Psychopathological and Cognitive Effects of Therapeutic Cannabinoids
Why is this important to me?
Cannabinoids, which are the chemical components of marijuana, may help improve spasticity and chronic pain if you have MS. However, the side effects that may be associated with therapeutic use of cannabinoids have not been thoroughly explored. Such side effects may include abuse, withdrawal, anxiety, fatigue, cognitive problems, psychotic problems, and others.
What is the objective of this study?
The authors assessed side effects of cannabinoid therapy by enrolling 17 people with MS who had never taken cannabinoids. The participants were divided into two groups and were given a mixture of two cannabinoids that was sprayed underneath the tongue or an indistinguishable placebo. Participants were allowed to spray the compound as many times as they needed to subjectively relieve spasticity. Half the participants received cannabinoids and the other half received a placebo. After 3 weeks, all participants entered a 2-week washout period, and then switched to the other formulation (placebo or cannabinoid spray) for another 3 weeks. The authors assessed fatigue, anxiety, psychopathology, cognitive function, abuse tendencies, withdrawal symptoms, and the physical and psychological impact of MS on quality of life. Results showed:
- No significant differences were found in any of these above variables when comparing post-placebo to post-cannabinoid administration.
- No serious adverse events, abuse, or withdrawal symptoms were found.
- A positive correlation was found between blood levels of one of the cannabinoid components and psychopathological factors such as sensitivity, aggressiveness, and paranoia.
- One participant experienced strong desire to resume cannabinoids 2 weeks (but not 2 months) after treatment ended.
- A tendency towards drowsiness and slower thinking was found in the group taking cannabinoids.
- This study did not evaluate whether cannabinoid use was effective for reducing spasticity or relieving any other MS symptoms.
In conclusion, the results of this study suggest that short-term use of cannabinoids will not impact your physical symptoms of MS, such as arm and leg function or fatigue. Short-term use of cannabinoids does not induce psychological problems, abuse, or withdrawal in most people. However, higher doses or longer exposure to cannabinoids could lead to such problems. Indeed, other studies have suggested that long-term recreational use of cannabinoids leads to slower cognitive processing. Careful evaluation of a history of psychosis should be done if you are considering taking cannabinoids for your MS, as individuals with such a history may be more susceptible to experiencing psychotic side effects. Because findings have varied across studies, additional studies with larger numbers of participants are needed.
How did the authors study this issue?
The authors enrolled 17 people with MS who had never taken cannabinoids. Participants received placebo or cannabinoids, both in the form of a spray under the tongue. The cannabinoid spray contained equal amounts of two cannabinoids: D-9-tetrahydrocannabinol and cannabidiol. After 3 weeks of placebo or cannabinoids and a 2-week washout, participants switched to the other formulation (cannabinoids or placebo) for another 3 weeks. Participants answered questionnaires that were designed to assess fatigue, anxiety, cognitive problems, physical and psychological impact of MS on quality of life, psychotic problems, abuse, and withdrawal. Participants answered these questionnaires before beginning treatment, at the end of the placebo and cannabinoid treatment phases, and 2 weeks and 2 months after the study ended.
| SHARE: | |||||
Original Article
Psychopathological and Cognitive Effects of Therapeutic Cannabinoids in Multiple Sclerosis: A Double-Blind, Placebo Controlled, Crossover Study
Aragona, Massimiliano MD*; Onesti, Emanuela MD†; Tomassini, Valentina MD‡; Conte, Antonella MD‡; Gupta, Shiva MD§; Gilio, Francesca MD‡; Pantano, Patrizia MD‡; Pozzilli, Carlo PhD‡; Inghilleri, Maurizio PhD, MD‡
Clinical Neuropharmacology
Objectives: To study possible psychopathological symptoms and cognitive deficits, abuse induction, as well as general tolerability and effects on quality of life, fatigue and motor function in cannabis-naïve patients with multiple sclerosis (MS) treated with a free-dose cannabis plant extract (Sativex).
Methods: In an 8-week, randomized, double-blind, placebo-controlled, parallel group crossover trial, 17 cannabis-naïve patients with MS were assessed at baseline and at the end of the cannabis and placebo phases of the trial (each of 3 weeks) by means of Symptom Checklist-90 Revised, Self-rating Anxiety Scale, Multiple Sclerosis Functional Composite (of which 1 dimension is the Paced Auditory Serial Additional Test that was used to evaluate cognition), Visual Analogue Scale on health-related quality of life, Multiple Sclerosis Impact Scale-29, and Fatigue Severity Scale.
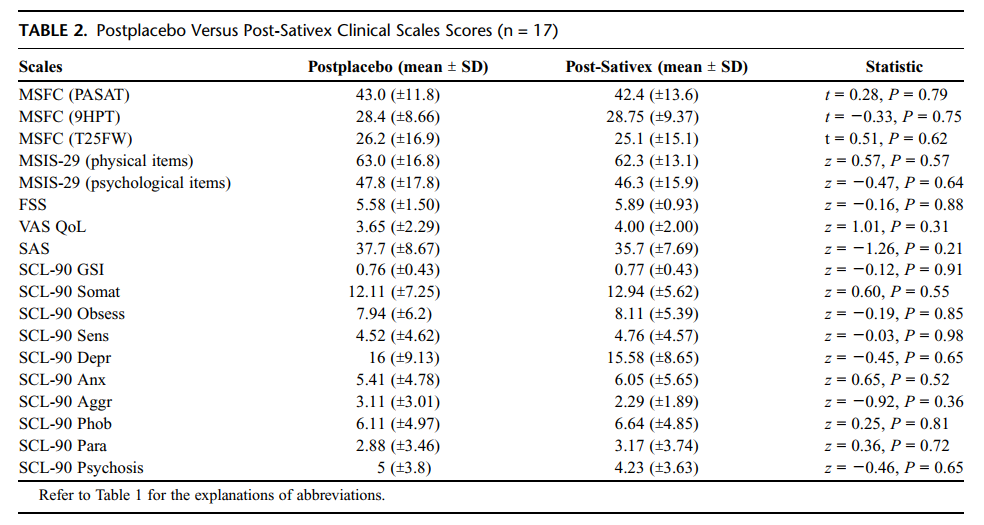
Results: Postplacebo versus postcannabinoid scores showed that no significant differences could be detected on all the variables under study. A significant positive correlation was found between Δ-9-tetrahydrocannabinol blood levels and scores at the General Symptomatic Index and at the "interpersonal sensitivity," "aggressive behaviour," and "paranoiac tendencies" subscales of the Symptom Checklist-90 Revised. No serious adverse events, abuse tendencies, or direct withdrawal symptoms were reported. Increased desire for Sativex with secondary depression was reported in 1 subject.
Conclusions: Cannabinoid treatment did not induce psychopathology and did not impair cognition in cannabis-naïve patients with MS. However, the positive correlation between blood levels of Δ-9-tetrahydrocannabinol and psychopathological scores suggests that at dosages higher than those used in therapeutic settings, interpersonal sensitivity, aggressiveness, and paranoiac features might arise, although greater statistical power would be necessary to confirm this finding.
Psychopathological symptoms and cognitive impairment are often reported as possible side effects of recreational cannabis use. In population surveys, anxiety and psychotic symptoms are commonly experienced after cannabis use.1,2 The psychotomimetic effect of cannabis, which was proved in experimental studies reporting transient positive and negative psychotic symptoms together with other psychopathological phenomena,3 is greater in subjects predisposed for psychosis4,5 and probably in adolescent smokers due to a greater vulnerability of the developing brain.6 On the other hand, cannabis use has been frequently reported in anxiety disorders7; it might precipitate psychosis,5,8 and the overall risk of developing psychotic symptoms has been described as increasing 3-fold in cannabis users.6
The acute administration of cannabis was reported to produce transient effects on several neuropsychological functions (eg, attention, memory, visual-motor coordination, verbal fluency, and psychomotor speed).3,9,10 In Bheavy users[ (eg, subjects smoking several joints per day, during periods of many years), a long-term, mild cognitive impairment was reported.11,12 Although no significant cognitive deficits were reported in frequent but moderate users of cannabis,13 the persistent effects of cannabis on cognition remain uncertain.14 Cannabinoids have recently been suggested for the treatment of neurodegenerative disorders, vascular and inflammatory diseases, cancer, and central pain,15 and they are currently suggested for the treatment of spasticity and chronic pain in multiple sclerosis (MS). In the light of this potential therapeutic role, studies focusing on psychological and cognitive tolerability of cannabinoids in MS are eagerly awaited.16 At the moment, some studies on general tolerability of cannabis treatment in MS are available,17 whereas, at least in our knowledge, no specific data have been published on its cognitive and psychopathological tolerability.
Consequently, the aims of this study were (i) to characterize the effects of a 3-week cannabinoid treatment with a cannabis plant extract containing Δ-9-tetrahydrocannabinol (Δ-9-THC) and cannabidiol (CBD) in equal proportions on several psychopathological dimensions, including psychotic symptoms and anxiety, and cognitive performances in cannabis-naBve patients with MS; (ii) to evaluate the tolerability and possible abuse induction; and (iii) to study the effects of cannabis on quality of lifeYrelated dimensions, on fatigue and motor function.
MATERIALS AND METHODS
Subjects
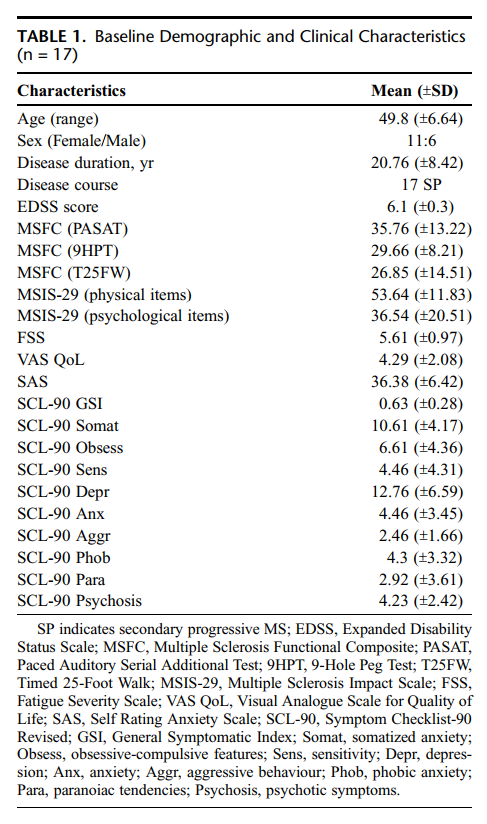
Seventeen patients with MS were recruited from the MS Outpatient Clinic at BSapienza[ University. After obtaining written informed consent, subjects underwent a baseline neurological and psychiatric assessment. To be eligible for inclusion into this study, patients had to fulfill the following criteria: male or female subjects between 18 and 60 years of age; right-handed with normal right-hand function; a baseline Expanded Disability Status Scale18 score ranging from 3.5 to 6.5; a stable disease for at least 30 days before study entry and nosystemic corticosteroid therapy within 4 weeks of randomization; significant spasticity in at least 2 muscle groups; antispastic and immunomodulatory agents (dosage, frequency, and route of administration) stable, before the study entry, for at least 1 and 6 months, respectively; no history of epilepsy of alcohol or substance abuse and no major medical illnesses; absence of psychiatric disorders or cognitive impairment at first evaluation; no history of psychiatric disorders; no concomitant therapy with psychoactive drugs; no female patient who was pregnant, lactating, or planning pregnancy during the course of the study; and no previous use of cannabis. This study was approved by the local Ethical Committee. Patients were informed about the potential for psychosis, anxiety, and panic, and they gave their informed consent to be included into the trial.
Study Drug
Patients received cannabis-based medicine extract (Sativex, GW Pharma, UK) presented in a pump action sublingual spray. Sativex is composed of whole cannabis plant extract, containing Δ-9-THC (27 mg/mL) and CBD (25 mg/mL), in ethanol/propylene glycol (50:50) excipient. Each actuation delivers 100 KL of spray, containing THC 2.7 mg and CBD 2.5 mg. Placebo had the appearance, smell, and taste of the active formulation in ethanol/propylene glycol (50:50) excipient but contained no active components.
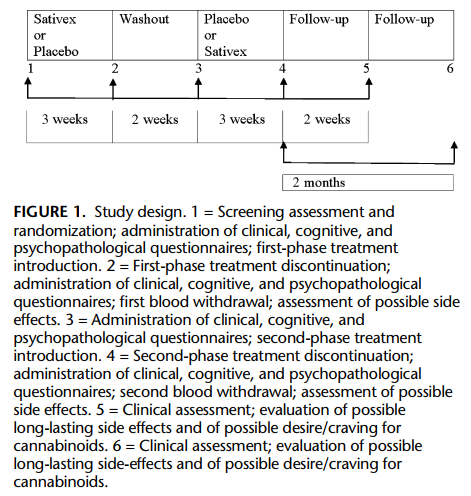
Study Design
This 8-week, randomized, double-blind, placebo-controlled, crossover trial was part of a larger study on functional magnetic resonance imaging and electrophysiological correlates of cannabinoid effect in MS patients experiencing spasticity (Pozzilli C et al, personal communication).
Patients taking immunomodulatory, antispastic, and/or any other pharmacological treatment were admitted in the study if therapy intake was stable for at least 6 and 1 months, respectively, before the study entry and were requested to maintain the intake of these pharmacological agents at fixed dosage, route, and time of administration. Trial assessments were scheduled at fixed times to be performed under standardized identical conditions (eg, daytime, interval to the last administration of antispastic, immunomodulatory compounds, and others).
Screening assessment was followed by randomization and dose introduction. Patients were randomly assigned to 2 counterbalanced groups starting either with Sativex or with placebo as the first drug. After 3 weeks, the first treatment was discontinued, and patients entered a washout phase of 2 weeks, before starting the second treatment phase of 3 weeks. During each treatment period, all patients had to reach the optimal, individualized dosage to subjectively relief spasticity. The number of daily actuations was recorded.
As shown in Figure 1, all patients completed the psychopathological and cognitive measures before starting therapy and at the end of both Sativex and placebo treatment phases. A withdrawal was done at the end of each treatment phase to evaluate the THC and CBD levels in the blood. Patients were recontacted and asked to estimate their desire for cannabis and whether they had noted any new medical or psychiatric problem twice after the end of the study, 2 weeks, and 2 months later, respectively.
Outcome Measures
At each visit, all patients were asked to complete rating scales to assess fatigue, disability, psychopathology, cognitive functioning, and the physical and psychological impact of MS on quality of life.
Specifically, psychopathological symptoms were assessed using 2 self-reporting devices: the Symptom Checklist-90 Revised (SCL-90-R)19 and the Self-rating Anxiety Scale (SAS).20 The SCL-90-R is a 90-item symptom inventory; the subject rates each item on a 5-point scale of distress, from 0 "not-at-all" to 4 "extremely." The 90 items are scored and interpreted in terms of 9 primary symptom dimensions and 1 global severity index of distress named General Symptomatic Index. The dimensions are labeled as somatization, obsessive-compulsiveness, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. The SAS is a 20-item symptom inventory specifically designed to study anxiety; the subject rates each item on a 4-point scale of distress, from 1 "none or a little of the time" to 4 "most or all of the time."
Cognitive performances were evaluated using the Paced Auditory Serial Additional Test (PASAT), 1 of the 3 dimensions of the Multiple Sclerosis Functional Composite (MSFC).21PASAT is a measure of cognitive function that assesses auditory information processing speed and flexibility, as well as calculation ability. The dependent variable is the sum of correct answers given by the subject (out of 60 possible). The other 2dimensions of the MSFC are the Timed 25-Foot Walk (T25FW), which is a quantitative measure of leg function and ambulation, and the 9-Hole Peg Test (9HPT), a quantitative measure of arm and hand function. To calculate the overall MSFC, scores for each component are converted to z scores using a specific formula. All these 3 independent clinical dimensions contribute equally to the overall MSFC score.
Quality of life and physical and psychological impact of MS from the patients' perspective were studied by means of 3instruments: the Visual Analogue Scale on health-related quality of life,22the Fatigue Severity Scale,23 and the 29-item Multiple Sclerosis Impact Scale.24 Visual Analogue Scale on health-related quality of life consists of a straight line of a specified length with verbal descriptors at each end consisting of short and easily understood phrases that describe the variable being measured. Fatigue Severity Scale is a 9-item questionnaire that assesses the effect of fatigue on daily living. Each item is a statement on fatigue that the subject rates from 1 "completely disagree" to 7 "completely agree." Patients with a mean score of 4 or more were defined as suffering from significant fatigue. The 29-item Multiple Sclerosis Impact Scale is an instrument measuring the physical (20 items) and psychological (9 items) impact of MS. The 2 summary scores are generated by summing individual items and then transformed to a 0 to 100 scale. High scores indicate worse health.
THC and CBD Plasma Measurement
Tetrahydrocannabinol and CBD levels in the plasma were measured according to the LC-MS/MS spectrometric method (Liquid Chromatography with tandem Mass Spectrometer detection) of Valiveti and Stinchcomb,25 with slight modifications. Chromatographic analyses were carried out using a High-Performance Liquid Chromatography with tandem Mass Spectrometer detection (HPLC-MS/MS) system. Negative electrospray ionization was used and all analyses were performed in Multiple Reaction Monitoring (MRM) mode. The transition were m/z 313-245 for THC and CBD. The limit of quantitation was 0.1 ng/mL for Δ-9-THC and CBD.
Statistical Analysis
We assigned patients to treatment sequence by using an independent statistician-generated randomization code. Investigators allocated patients consecutively by time of inclusion at the study site.
9HPT task periods were averaged over the 2 trials for each hand, averaging together both the dominant and nondominant hand trials. T25FW trial periods were averaged over the 2 trials. MSFC scores were calculated using test results from the baseline visit from all patients. Analyses for MSFC were conducted without replacing data values as well.
For all variables measured except for MSFC PASAT, 9HPT, and T25FW, Wilcoxon's matched pairs signed-rank tests were used to compare variables measured at the end of each 3-week treatment period (postplacebo vs post-Sativex) given that all variables except those listed above are defined by finite scoring systems that do not fit a normal distribution. For MSFC PASAT, 9HPT, and T25FW, paired t tests were conducted to compare the variables postplacebo versus post-Sativex levels, given that these 3 variables are defined by a continuous, normally distributed time scale.
For correlations with CBD and Δ-9-THC serum levels, all dependent variables except for MSFC PASAT, 9HPT, and T25FW measured during the post-Sativex period were correlated using Spearman rank correlations given the nonparametric nature of these variables as stated above. For MSFC PASAT, 9HPT, and T25FW values measured during post-Sativex periods, Pearson correlations were evaluated to correlate with CBD and Δ-9-THC serum levels.
All tests are reported with their local P values, thus identifying differences that would have potential statistical significance (P < 0.05), if chosen as the primary efficacy criterion. For the correlation statistics, Bonferroni correction was not used because the comparisons did not fall within a single joint family of comparisons by definition.
RESULTS
All the 17 patients who entered the study completed the trial and were included in the analysis.
Baseline demographic and clinical characteristics are reported in Table 1. Baseline assessment did not reveal significant cognitive problems. All the included patients had taken antispastic and immunomodulatory compounds in their clinical history, with generally poor results. During the study, only 5 patients were taking immunomodulatory compounds (interferon-beta, copolymer-1, or azatioprine) and 10 were taking antispastic drugs (baclofen, dantrolene sodium, chlorohydrate tizanidina, or botulinum toxin). All patients maintained the intake of these pharmacological agents at fixed dosages, route, and time of administration in relation to the assessment points of the trial.
The number of daily actuations used was significantly higher during the placebo than during the Sativex period (15.16 + 4.51 vs 8.20 + 3.15; t = 5.31, P < 0.001).
Postplacebo versus post-Sativex scores are reported in Table 2. It is shown that neither psychopathological nor cognitive scores differed between the Sativex and the placebo period. Although objective measures did not reveal any cognitive impairment, 11 patients during the Sativex administration and 2 subjects during the placebo period reported subjective drowsiness and sense of "slower-thinking"; 1 of the 11 subjects who reported this symptom experienced transient mental confusion with temporal and spatial disorientation. Measures of fatigue, leg and arm function, quality of life, and physical and psychological impairment due to MS did not differ between the 2 treatment periods.
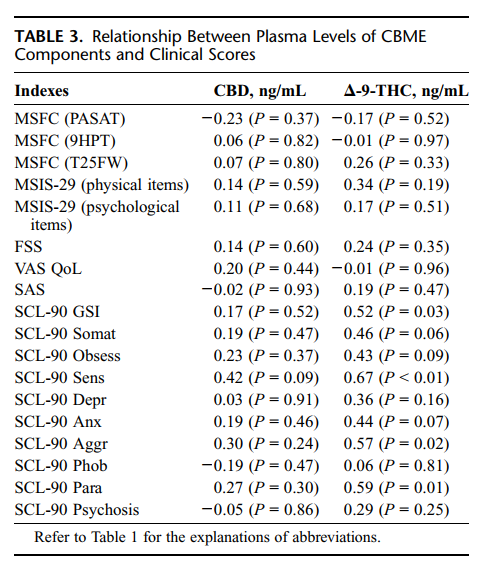
Correlation between clinical scores and plasma levels of CBD and Δ-9-THC are reported in Table 3. A significant positive correlation was found between the Δ-9-THC blood levels and scores at the General Symptomatic Index and at the "Interpersonal sensitivity," "Aggressive behaviour," and "Paranoiac tendencies" of the SCL-90-R. No correlations were found between the other major component of Sativex (CBD) and the studied variables.
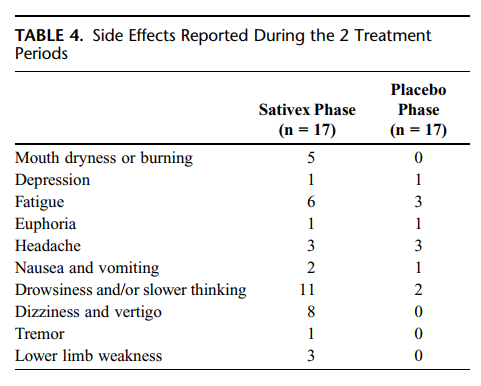
No serious adverse events (hospitalization, death) occurred during the study. Side effects reported during the placebo and Sativex phases were generally mild (Table 4). During the Sativex administration, 1 patient required treatment in the emergency department because of transient mental confusion with temporal and spatial disorientation, tachycardia, increased blood pressure, and mydriasis. Symptoms recovered in few hours without requiring hospitalization, and the patient was able to continue the study without further problems.
All patients were recontacted twice after the end of the study, 2 weeks, and 2 months later, respectively. At the 2-week but not at the 2-month visit, one 56-year-old lady reported intense desire to reintroduce Sativex after withdrawal and developed depressive symptoms (successfully treated with sertraline 50 mg/die), probably related to the impossibility to keep taking Sativex. None of the other patients reported craving for cannabis, dependence, and withdrawal effects, as well as any new medical or psychiatric problems.
DISCUSSION
The primary aim of this study was to explore the onset of psychopathological symptoms and cognitive deficits in cannabis-naïve patients with MS treated with a cannabis plant extract (Sativex) for relieving their spasticity. General tolerability, possible abuse induction, and the effects of cannabinoids on quality of life, fatigue, and motor function were also studied.
Our study evaluated the effects of cannabinoids on cognition by means of PASAT, which is widely used to test sustained attention, divided attention, concentration, and information processing speed in MS patients.26 We found no significant differences between Sativex and placebo in the PASAT scores, although we found a higher frequency of subjective drowsiness and sense of "slower-thinking" in the cannabinoid phase of the study. Tacking into consideration the high sensitivity of PASAT for revealing cognitive deficits, we are prone to interpret this finding as of no significant impact on cognition in our cohort. Although a previous study reported an improvement on PASAT performances during a period of 2 weeks,27 our results are in accordance with a 4-week study, which did not find any difference compared with placebo.28Our findings support the hypothesis that cannabinoids taken as a treatment for MS symptoms do not affect cognition, at least in the short term. Note that research on long-term recreational cannabis users (mean length of regular use >10.3 years) showed that slower cognitiveprocessing rates in PASAT performances are more common among long-term rather than short-term users,11 suggesting that although cognitively safe, a note of caution is needed in the long-term administration. Another aspect to be considered as of potential impact on cognition is the dosage. Our findings suggest that in the range of therapeutic dosages (mean daily puffs 8.20 + 3.15, approximately corresponding to 22 mg of Δ-9-THC), cannabinoids do not significantly affect cognition, and this is in accordance with results from a study reporting no significant cognitive deficits in frequent but moderate users of cannabis.13 Although we did not find any significant correlation between Δ-9-THC and CBD plasma levels and PASAT scores at the end of the Sativex treatment, which suggests that at these moderate dosages, there is no dose effect on cognition; cognitive tolerability at higher dosages cannot be predicted on the basis of our data because the study was not designed to detect such an effect.
The other major element under study was the effect of cannabinoid treatment on psychopathology. Psychopathological symptoms were often found in recreational cannabis users,1,2 and cannabis use had been frequently reported in anxiety7 and psychotic disorders.29Our finding that psychopathological scores did not differ between cannabinoids and placeboapparently contrasts with the report of transient "positive" and "negative" psychotic symptoms, perceptual alterations, euphoria, and anxiety in healthy volunteers.3 However, in that study, the authors evaluated the acute effect of 2 fixed dosages of intravenously injected Δ-9-THC in subjects who had previously smoked cannabis, whereas in our study patients were cannabis naïve, they took a free-dosage oral-administered cannabis extract that also contains CBD, and the effects on psychopathology were evaluated after 3 weeks of treatment. These differences in the study design make a direct comparison of these 2 studies difficult. To our knowledge, there was no previous study specifically focused on the evaluation of psychopathological phenomena in MS patients. However, a few psychiatric disturbances were reported among other side effects. In particular, euphoria was the most frequently reported symptom, ranging from 3.2% to 26.6% of the treated subjects,30-34 whereas other phenomena were found sporadically (hyperactivity,30 dissociation,32 feeling "paranoid," and hallucinations34).
All patients were recontacted twice after the end of the study, 2 weeks, and 2 months later, respectively. At the 2-week but not at the 2-month visit, one 56-year-old lady reported intense desire to reintroduce Sativex after withdrawal and developed depressive symptoms (successfully treated with sertraline 50 mg/die), probably related to the impossibility to keep taking Sativex. None of the other patients reported craving for cannabis, dependence, and withdrawal effects, as well as any new medical or psychiatric problems.
In our study, euphoria was reported only by 1 patient in the treatment group (5.8%), and its rate was comparable to that reported in the placebo group. This finding does not differ considerably from previous studies. However, neither our nor previous studies used standardized questionnaires to measure euphoria, and further studies with more objective and sensitive measures are thus required.
Having found no differences in the SCL-90 and SAS scores, our study suggests that in the short term, Sativex administered to cannabis-naïve patients for therapeutic purposes does not increase the risk of main psychopathological symptoms. However, we found a moderate but significant positive correlation between the plasma levels of Δ-9-THC (the principal active ingredient of cannabis) and some of the SCL-90 scales. This suggests that although at the low therapeutic levels used in this study, cannabinoids do not induce the onset of psychopathological symptoms, at higher dosages, the risk of worsening interpersonal sensitivity (eg, the tendency of experiencing feeling of inadequacy in the relationship with others), aggressiveness, and paranoiac thoughts cannot be excluded. This possibility, together with previous reports of sporadic symptoms like feeling "paranoid" and hallucinations34 in patients with MS treated with cannabinoids, suggests that a careful evaluation of history of psychosis should be done in all patients addressed to cannabinoids treatment. Finally, the correlation between Δ-9-THC and aggressiveness found in our study contrasts with a previous research that excluded aggressive feelings in 50 MS patients treated with cannabis-derived drug.31Considering that the relationship between cannabis use and aggressiveness is still debated,35,36 future studies on larger samples of patients taking cannabinoids as a therapy are needed.
Another aim of the study was the evaluation of the impact of cannabinoid treatment on some physical problems related to MS (eg, reduced leg and arm function, fatigue), as well as on quality of life and physical and psychological impairment. Our findings suggest that in the short term, none of these variables changed significantly. These results are in line with current literature showing that administered for a 2- to 4-week treatment period, cannabis does not significantly improve arms and legs mobility27 and fatigue,37 whereas they differ from those of Killestein and colleagues,37 who found a significant worsening in the total MSFC and 9HPT scores. Our findings also confirm previous studies showing how cannabis use did not covary with general scores on quality of life,37-39 whereas a positive slight trend benefit was found on legs motility (T25FW) in long-term studies.39 More promising results have been previously reported with subjective reports: cannabis was reported to relief fatigue and dysfunction of walking and balance and to improve emotional dysfunction.40 However, the fact that findings with objective measurements are mostly conflicting suggests that studies on larger cohorts are needed to evaluate the impact of cannabinoid treatment on these variables.
Another issue to be evaluated was the general tolerability. All subjects included in the studycompleted the 3 weeks of trial, and no serious adverse events occurred during the study. Side effects were slightly more frequent during active treatment than during placebo. No clinically relevant changes were observed in physical examinations, with the exception of transient increased blood pressure in 1 patient. No changes were found in hematology or biochemistry parameters. Finally, although some possible effects of Sativex are similar to those reported with antispastics (eg, drowsiness, mouth dryness, dizziness, vertigo, and fatigue) and consequently could have been responsible of a summatory effect, this was not found in our study.
Taken together, these findings suggest that Sativex was well tolerated. Long-term studies confirm the good tolerability profile of cannabinoids for therapeutic use.17,39
Finally, a crucial point that is usually a matter of concern when the therapeutic use of cannabinoids is proposed is the possibility that cannabis may induce dependence and abuse. At this regard, our study was in a good position to observe these possible phenomena, being on cannabis-naïve subjects and allowing for free dosages. Interestingly, none of the patients abused the use of Sativex during the treatment phase, the mean daily dosage being quite low (23-25 mg) and the number of daily actuations being significantly higher in the placebo than in the cannabis phase (suggesting that when patients had reached the therapeutic daily dosage, they spontaneously interrupted the dosage augmentation of Sativex without craving induction, whereas in the case of placebo, they continued to increase the number of daily actuations trying to reach active effects). Different motivations of the user (recreational vs therapeutic) might partially explain our findings that cannabinoid treatment in MS does not induce tolerability and dosage increase. However, in the case of other psychoactive substances (eg, benzodiazepines, tramadol, and zolpidem), some tendency to induce tolerability is also found in therapeuticsettings with consequent increased risk of dosage augmentation and dependence,41-44suggesting that our findings may be not com pletely due to the above-mentioned factor. A related but different factor is that in recreational use of cannabis, the route of administration (inhalation) usually maximizes the Cmax and shortens the Tmax in order to increase the psychoactive effect, whereas Sativex (sublingual/oromucosal administration) reaches a low Cmax and has a fairly long Tmax.45 Different pharmacokinetics might be responsible for less gratifying subjective experiences due to the delayed psychoactive effect of Sativex, thus explaining (at least in part) why patients were not driven to increase the dosage.
Furthermore, direct withdrawal symptoms were not reported, as expected.17 However, some days after the end of the trial, 1 patient experienced desire to reintroduce Sativex. Whether this desire was driven by a perceived effective treatment (she explained it as a reaction to the impossibility to continue the treatment that she considered very helpful, and in effect, she presented a significant leg mobility improvement, with a 57% drop of T25FW scores) or whether it represented a withdrawal symptom cannot be addressed by a single case. Two months after the termination of the study, all the subjects included in the study had not increased desire to be treated again with Sativex and did not present any new medical or psychiatric problem. However, long-term studies are needed in larger samples to evaluate the possible dependence induced by cannabinoid therapy.
There are some limitations in our study, which need to be considered in the interpretation of our findings. The sample size was quite small to address completely all the questions arising from a study evaluating the psychopathological impact of cannabinoids. However, the power of the study was though to evaluate the effect of Sativex on physical symptoms (eg, spasticity) and its electrophysiological and imaging counterpart. The study duration of treatment was relatively short, again aimed to provide pilot information about the study drug. As a consequence, direct generalization of these data to larger clinical populations is not viable, and more focused studies are needed to investigate the safety of cannabinoids on cognitive and psychopathological domains in patients with MS. Cognitive performances were studied with 1 test (PASAT), and no other information about cognition in this cohort was available. More detailed test batteries should be included in future studies. Finally, the oromucosal route of administration of cannabis might have induced unpredictable interindividual and intraindividual variability in plasma Δ-9-THC levels, not detectable with blood tests.
In conclusion, cannabinoids prescribed for therapeutic reasons to cannabis-naïve patients with MS did not induce onset of psychotic or anxiety symptoms and did not impair cognition. Safety and tolerability were generally good, drug tolerance and dose increasing were not reported during the trial, and desire for Sativex or abuse was not present at follow-up. However, the positive correlation between blood levels of Δ-9-THC and psychopathological scores suggests that at dosages higher than those used in therapeutic settings, interpersonal sensitivity, aggressiveness, and paranoiac features might arise. Finally, the effects of cannabinoids on quality of life, fatigue, and motor function of MS patients were nonsignificant, suggesting that the effective impact of cannabinoids in these patients as well as the most proper target symptoms remain to be better ascertained.
ACKNOWLEDGMENTS
Sativex was kindly provided by GW Pharma Ltd, Salisbury, England. The grant has been supported as the Project of University Research (ex-quota 60%)-year 2004-by the University "Sapienza" of Rome.
REFERENCES
1. Thomas H. A community survey of adverse effects of cannabis use. Drug Alcohol Depend 1996;42:201Y207.
2. Stefanis NC, Delespaul P, Henquet C, et al. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction 2004;99:1333Y1341.
3. D_Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effect of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 2004;29: 1558Y1572.
4. Henquet C, Krabbendam L, Spauwen J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2005;330:11.
5. Degenhardt L, Hall W. Is cannabis use a contributory cause of psychosis? Can J Psychiatry 2006;51:556Y565.
6. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: a systematic review. J Psychopharmacol 2005;19:187Y194.
7. Dannon PN, Lowengrub K, Amiaz R, et al. Comorbid cannabis use and panic disorder: short term and long term follow-up study. Hum Psychopharmacol 2004;19:97Y101.
8. Iversen L. Long-term effects of exposure to cannabis. Curr Opin Pharmacol 2005;5:69Y72.
9. Curran HV, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychoparmacol (Berl) 2002;164:61Y70.
10. O_Leary DS, Block RI, Turner BM, et al. Marijuana alters the human cerebellar clock. Neuroreport 2003;14:1145Y1151.
11. Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002;287: 1123Y1131.
12. Messinis L, Kyprianidou A, Malefaki S, et al. Neuropsychological deficits in long-term frequent cannabis users. Neurology 2006;66: 737Y739.
13. Jager G, Kahn RS, Van Den Brink W, et al. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacol (Berl) 2006;185:358Y368.
14. Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, et al. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychol Rev 2004;14:1Y41.
15. Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs 2000;60:1303Y1314.
16. Killestein J, Uitdehaag BM. Cannabinoids in multiple sclerosis: urgent need for long term trials. J Neurol Neurosurg Psychiatry 2005;76:1612.
17. Wade DT, Makela PM, House H, et al. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 2006;12:639Y645.
18. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444Y1452.
19. Derogatis LR. The SCL-90-R: Administration Scoring and Procedures Manual II. Baltimore: Clinical Psychometric Research; 1992.
20. Zung WWK. A rating instrument for anxiety disorders. Psychosomatics 1971;12:371.
21. Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999;5:244Y250.
22. Parkin D, Rice N, Jacoby A, et al. Use of a visual analogue scale in a daily patient diary: modelling cross-sectional time-series data on health-related quality of life. Soc Sci Med 2004;59:351Y360.
23. Herlofson K, Larsen JP. Measuring fatigue in patients with Parkinson_s diseaseVthe Fatigue Severity Scale. Eur J Neurol 2002;9:595Y600.
24. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001;124:962Y973.
25. Valiveti S, Stinchcomb AL. Liquid chromatographic-mass spectrometric quantitation of Delta9-tetrahydrocannabinol and two metabolites in pharmacokinetic study plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci 2004;803:243Y248.
26. Rosti E, Hamalainen P, Koivisto K, et al. The PASAT performance among patients with multiple sclerosis: analyses of responding patterns using different scoring methods. Mult Scler 2006;12:586Y593.
27. Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 2004; 10:417Y424.
28. Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812Y819.
29. Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. Br J Psychiatry 2005;187:306Y313.
30. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;31:253.
31. Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 2004;10:417Y424.
32. Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812Y819.
33. Collin C, Davies P, Mutiboko IK, et al. Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 2007;14: 290Y296.
34. Chong MS, Wolff K, Wise K, et al. Cannabis use in patients with multiple sclerosis. Mult Scler 2006;12:646Y651.
35. Monshouwer K, Van Dorsselaer S, Verdurmen J, et al. Cannabis use and mental health in secondary school children. Findings from a Dutch survey. Br J Psychiatry 2006;188:148Y153.
36. Arendt M, Rosenberg R, Fjordback L, et al. Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychol Med 2007;37:935Y945.
37. Killestein J, Hoogervorst EL, Reif M, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002;58:1404Y1407.
38. Ventegodt S, Merrick J. Psychoactive drugs and quality of life. Sci World J 2003;18:694Y706.
39. Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005;76:1664Y1669.
40. Consroe P, Musty R, Rein J, et al. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol 1997;3:44Y48.
41. Khong E, Sim MG, Hulse G. Benzodiazepine dependence. Aust Fam Phys 2004;33:923Y926.
42. Simon GE, Ludman EJ. Outcome of new benzodiazepine prescriptions to older adults in primary care. Gen Hosp Psychiatry 2006;28:374Y378.
43. Brinker A, Bonnel RA, Beitz J. Abuse, dependence, or withdrawal associated with tramadol. Am J Psychiatry 2002;159:881.
44. Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol 2000;23: 281Y283.
45. Guy GW, Robson PJ. A phase 1, open-label, four way crossover study to compare the pharmacokinetic profiles of a single dose of 29mg of a cannabis-based medicine extract (CBME) administered on 3 different areas of the buccal mucosa and to investigate the pharmacokinetics of CBME per oral in healthy male and female volunteers. J Cannabis Ther 2003;3/4:121Y152.