The Emerging Role of the Gut Microbiome
Why is this important to me?
This article may be frustrating for some readers, because it poses many more questions than it answers. It is still worth reading, however, because it sheds some light into an area of increasing interest to healthcare professionals — the largely unexplored world of the gut microbiome.
All of us are home to thousands of types of bacteria in our gastro-intestinal tract. These bacteria help to ensure that we metabolize and draw energy from the foods we eat, protect us from intruding bacteria and viruses, and keep our digestive system in good working order. This multitude of bacterial types, which we refer to as the “gut microbiome,” maintains a delicate balance in order to function well: increase or decrease one type of bacteria, and the entire digestive tract may be disrupted.
Our gut microbiome is increasingly of interest to healthcare professionals because of mounting evidence that disruption of the gut microbiome (the term is “dysbiosis”) can impact the other functions and well-being outside our digestive tract. Evidence exists, for example, that dysbiosis can reduce our ability to form new blood vessels and increase the severity of osteoporosis.
The authors of this article decided to examine whether dysbiosis may be part of the MS disease process. Certainly, a connection exists between our digestive tract and our brain and central nervous system: anyone who has experienced stress or anxiety understands this connection all too well. Evidence may suggest, however, that our gut may in turn exert immunologic and hormonal influence over our central nervous system.
What is the objective of this study?
The authors reviewed six earlier studies to examine the level of evidence that the gut microbiome and dysbiosis may play a role in the MS disease process. All studies except one confirmed that dybiosis was present in individuals with MS. Here, however, is where the authors found themselves faced with questions that prevented them from drawing further conclusions. These questions include:
- Does MS itself cause dysbiosis, or does dysbiosis contribute to the severity of symptoms that individuals with MS experienced?
- Did certain disease-modifying therapies in MS that the studies’ subjects take contribute to their dysbiosis?
- Did Vitamin D supplements that some study subjects took contribute to their dysbiosis?
- Does race and ethnicity play a role in the development of dysbiosis?
At this point, only additional future studies will help to answer these questions.
In the meantime, this article points to the continuing necessity of making wise choices with respect to your gut health. Diets rich in fiber are much more likely to support your gut microbiome than will a diet that relies on processed foods. If you would like to investigate other probiotic supplements, ask your healthcare provider for suggestions on how you can help to support your gut health.
| SHARE: | |||||
Original Article
The Emerging Role of the Gut Microbiome in Adult Patients With Multiple Sclerosis
Journal of Neuroscience Nursing
Pamela K. Newland, Margaret Heitkemper, Yanjiao Zhou
Background: Approximately 2.3 million people worldwide are currently living with multiple sclerosis (MS). The pathophysiologic mechanism of MS is not well known. It has been suggested that alterations in the normal gut flora may contribute to MS etiology and symptoms. Objective: The aims of this review are to describe the data suggesting a role for the gut microbiome in MS research and address its implications for practice.
Methods: A literature search of the following databases (PubMed, CINAHL, Cochrane library database, MEDLINE, Scopus, and Psychology and Behavioral Sciences) was conducted to find published studies relevant to gut microbiome in patients with MS. Study Selection: Five articles met the inclusion criteria of research studies of human gut microbiome in adults in English language and those receiving disease-modifying medications. Exclusion criteria were case reports and reviews.
Results: Human studies found that the gut microbiome was different among patients with MS, patients with MS who were treated with glatiramer acetate, and healthy controls. Discussion: There is beginning evidence to suggest that the gut microbiota is related to autoimmunity and the pathology of MS. However, more research is necessary to clarify these mechanisms.
Implications for Practice: A better understanding of the role of the gut microbiota in MS may lead to the development of targeted individualized interventions affecting the gut microbiota. These interventions may emphasize symptom self-management strategies such as diet.
Multiple sclerosis (MS) is a chronic autoimmune disease, characterized by areas of inflammation, demyelination, and neurodegeneration in the central nervous system (CNS) (Tremlett, Zhao, Rieckmann, & Hutchinson, 2010). It is believed that autoreactive lymphocytes mount abnormal responses against CNS autoantigens, although the exact immunopathology is not fully understood. Potentially autoimmune active T cells produce inflammation and axonal loss in the brain, spinal cord, and optic nerves. According to the National MS Society (n.d.; NMSS.org), risk factors include genetics, age, geography and ethnic background, and sex. Common clinical features of MS include motor, sensory, cognitive, and vision impairment; fatigue; and visceral organ sphincter dysfunction (Newland, Fearing, Riley, & Neath, 2012).
Recent studies (Berer & Krishnamoorthy, 2012; Sommer & Bäckhed, 2013) suggest complex linkages between the gut microbiota and CNS physiology that may affect neurological and immune functions (Yang, Nossa, & Pei, 2014). Recent advances reveal that thousands of bacteria, viruses, and, in some instances, helminths reside in and/or on the human body (Human Microbiome Project Consortium, 2012). They are collectively referred to as the microbiome. The interaction of microbiota with the host is essential in maintaining homeostasis in healthy individuals. Dysbiosis, or disruption of balance (Yang et al., 2014) of the human microbiome, is associated with a surprising range of local and systemic diseases or conditions that affect metabolism, vessel formation, and bone reabsorption (Sommer & Bäckhed, 2013). The cross talk between the gut microbiome and the brain is bidirectional. The gut microbiome may exert its impact on the CNS via neural, immunological, endocrine, and metabolic pathways. Stress and emotions can influence the gut microbiome through stress hormones such as cortisol and neurotransmitters (Figure 1, Supplemental Digital Content 1, available at http://links.lww.com/JNN/A79).
Interestingly, a growing body of evidence from both rodent and human studies suggests that the gut microbiomemay be involved in the pathogenesis of MS. For example, 2 studies using the experimental autoimmune encephalomyelitis (EAE) mouse model that mimics MS (Berer & Krishnamoorthy, 2012; Lee, Menezes, Umesaki, & Mazmanian, 2011) tested whether the commensal gut microbiota is related to extraintestinal immune function. The EAE animal model of MS is mediated by the activation of myelin-specific autoreactive T-helper cells. In addition, 3 studies examined the effects of broad-spectrum oral antibiotics versus intraperitoneal antibiotic administration on the development of EAE (Ochoa-Repáraz et al., 2009, 2010; Ochoa-Repáraz, Mielcarz, Begum-Haque, & Kasper, 2011). Two rodent studies explored probiotic therapy using Lactobacillus strains (eg, Bifidobacterium infantis family) (Takata et al., 2015). These studies found that gut microorganisms (in particular B. rodentis strain) can reduce the severity of EAE (Ezendam, de Klerk, Gremmer, & van Loveren, 2008; Ochoa-Repáraz et al., 2007, 2010; Takata et al., 2011; Wang et al., 2014). The Bifidobacterium strain is a Gram-positive, nonmotile anaerobic bacterium. Ecologically, this bacterium is found in food, sewage, and oral cavities but most often in the gastrointestinal tract of humans and rodents (B. bottacini).
At this time, there is limited work related to gut dysbiosis and symptoms or disease progression in humans with MS. Dysbiosis may be one of several factors involved in the pathogenesis and progression of MS (Foster & McVey Neufeld, 2013; Joscelyn & Kasper, 2014). Understanding these factors, their interactions, and how they may be involved in MS pathophysiology and symptom burden is a key component to educating the patient and family about the disease, as well as potential therapeutic approaches. The review examined the emerging clinical evidence supporting a role for gut microbiome in patients with MS and implications for nursing practice.
Methods
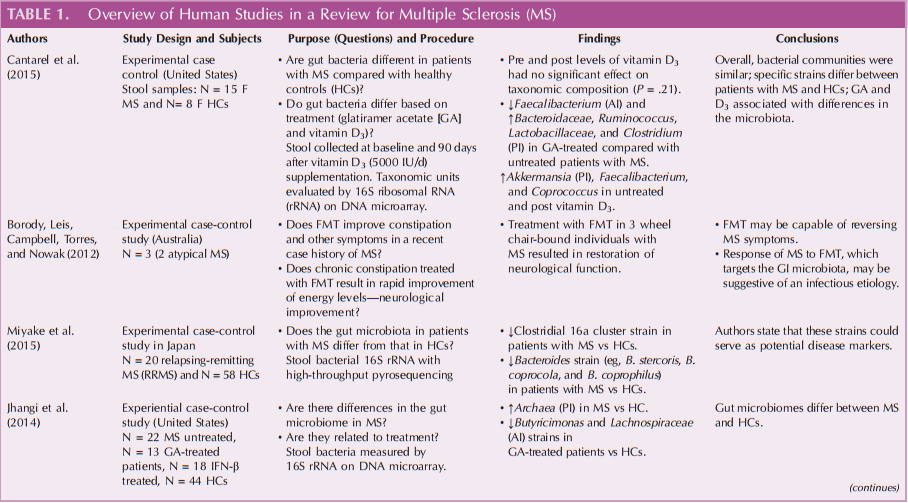
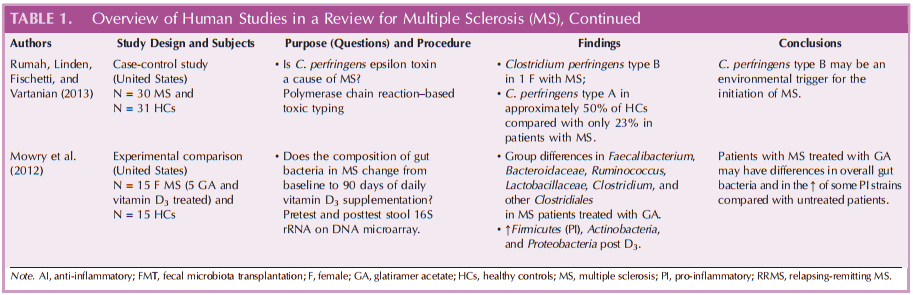
The literature search for this article was undertaken in PubMed, CINAHL, Cochrane library database, MEDLINE, Scopus, and Psychology and Behavioral Sciences. Key terms used were “gut microbiome,” “microbiota,” “multiplesclerosis,” and “microbiota” for the search. Review of abstracts and, if suitable, hand-searches by ancestry for further relevant articles were performed. Initial results netted more than 63 research articles. The search was completed in 2015, and only peer-reviewed research articles published after 2006 and in English language were considered. Those related to diet intervention and fecal transplantation were also included. Articles based on rodent model, commentary, or systematic review were excluded. Of these, 5 human studies were included for the review and are described and summarized in Table 1.
Results
Of the 5 studies reviewed, all agreed that gut microbiome dysbiosis is present in patients with MS (Table 1). Most studies were conducted in the United States, with the exception of 1 Japanese study (Miyake et al., 2015). Most described some differences in the presence of certain bacterial families (Butyricimonas strains, Faecalibacterium, Bacteroidaceae, Ruminococcus, Lactobacillaceae, Clostridium, and other Clostridiales) in patients with MS when compared with age-matched healthy controls (HCs). Specifically, the levels of bacterial strains from the Bacteroidaceae family were decreased in those with MS who were treated with glatiramer acetate (GA), whereas certain strains (eg, Faecalibacterium) were increased in untreated people with MS.
Recently, in a cross-sectional design, Cantarel et al. (2015) examined the gut microbiota of 15 white women with both MS and vitamin D insufficiency and 15 HCs. The subjects with MS were either untreated or receiving GA. Stool samples were taken 90 days after vitamin D supplements were administered. They found that the Faecalibacterium strain was lower in patients with MS treated with GA as compared with HCs. Similarly, von Geldern and Mowry (2012) found that GA-treated patients with MS showed community composition differences when compared with untreated patients with MS. Specifically, strains of Bacteroidaceae, Faecalibacterium, Ruminococcus, Lactobacillaceae, Clostridium, and other Clostridiales were lower compared with untreated patients with MS who had an increase in the Akkermansia, Faecalibacterium, and Coprococcus strains after vitamin D supplementation.
In a recent study in Japan, Miyake et al. (2015) identified 20 patients with relapsing-remitting MS and 50 HCs to compare strains of the gut microbiota. The investigators found that those with MS had significantly lower strains of Clostridia, Bacteroides, B. stercoris, B. coprocola, and B. coprophilus when compared with HCs.
Likewise, Rumah et al. (2013) examined the relationship of gut microbiome in a case-control study. They found that Clostridium perfringens type B strain was present in 1 case, whereas Clostridium perfringens type A strain was present in approximately 50% of HCs compared with only 23% in patients with MS.
Other differences in specific microbiota were described by Jhangi et al. (2014) who found an increase in Archaea strain in patients with MS versus HCs (P < .00001). In addition, the authors found that 2 taxa (Lachnospiraceae) (Table 1) were lower in patients with MS versus HCs. Both of these increased after treatment with interferon β or GA (Table 1).
In a recent case study analysis, Borody, Leis, Campbell, Torres, & Nowak (2012) studied fecal microbiota transplantation (FMT). In a case of a 29-year-old man with “atypical” MS who received 10 days of FMT, the investigator found improvement of constipation, as well as neurological symptoms (paresthesia and leg weakness). In this case, 3 years of FMT resulted in normal gastrointestinal, motor, and urinary function. Many unanswered questions remain, including FMT methodology, which includes best method of administration, what makes a “good donor,” (Borody, Paramsothy, & Agrawal, 2013), safety issues, and long-term effects of FMT.
Discussion
Evidence from the studies reviewed is that patients with MS, as well as those receiving disease-modifying therapy, were found to have differences in microbiome compared with HCs. The clinical significance of these differences is not known because of the small samples, and therefore, additional studies are needed with larger sample sizes. On the basis of the literature review, we identified that bacteria from Bacteroidetes, Firmicutes, Actinobacteria, and Akkermansia differ in persons with MS when compared with HCs. Yet, identification of how each of these strains impacts the mechanism of MS or symptoms has not been determined.
It has been suggested that supplementation with prebiotics/probiotics may be useful in maintaining microbiota balance (Riccio & Rossano, 2015). In addition, studies have shown that probiotic treatment improves severity of the mouse MS model induced with EAE (Ezendam et al., 2008). For example, Lactobacillus farciminis and B. animalis were found to both reduce MS symptom onset (Ezendam et al., 2008) and improve commensal microbiota (Lavasani et al., 2010) in the EAE model. Because these results were found in a rodent model, further research is needed to confirm that results are reproducible in people with MS. Although rodent studies indicate that prebiotics/probiotics are effective in reducing disease activity and improving dsybiosis, the use of prebiotics/probiotics in humans with MS requires further investigation.
Diet influences gut microbiota metabolism, as well as the amount and types of bacteria present in the gut (Riccio & Rossano, 2015). There is some evidence to support the possibility of symptom mitigation by increasing the amount and diversity of beneficial bacteria. For example, people who eat a diet high in fiber (prebiotic) are more likely to have more diverse and numerous microbiota than people whose diets include more processed foods (Riccio & Rossano, 2015).
Limitations
There are several limitations in this review. First, it is unknown whether dysbiosis is a result of MS disease itself or the treatment of disease-modifying therapy or vitamin D. Second, from the data presented, it is unclear whether the dysbiosis of the gut microbiome is the cause or result of MS. Third, in 2 studies (Cantarel et al., 2015), vitamin D was also taken, leading to the question of whether the results would be different if the patients had not had vitamin D insufficiency at baseline. In addition, not all studies were consistent with measuring stool samples both at baseline and after the intervention. Fourth, self-reported race and ethnicity were used, rather than genetic ancestry markers to attempt to minimize heterogeneity. Larger studies may want to consider using such markers in the screening or analysis process to account for differences in host genetics that may influence a person’s microbial colonization but may also need to consider self-reported cultural groupings so as to account for differences. Finally, the studies used metagenomic approaches, but a far more expansive study of the metabolic pathway, virus, and fungi is needed with a larger sample size. Likewise, many of the studies were composed of small samples of various types of MS (eg, relapsing-remitting MS vs secondary progressive MS) and conducted in observation environment, and not all used an HC group. Longitudinal experimental design is desired to capture the dynamics of gut microbiome in patients with MS.
Implications for Nursing Practice
Bringing the role of the gut microbiome to the forefront of nursing care and patient education may assist in the ongoing clinical practice for patients with MS. Several novel interventions may directly affect the microbiome in people with MS, although further research is needed to confirm these results from both rodent and humans with MS. One diet intervention that theoretically affects the microbiome is the administration of prebiotics and probiotics, such as Lactococcus lactis (Takata et al., 2015). Another recent discovery is the use of intermittent calorie restriction. Specifically, people with MS would restrict calories (ie, 500 calories, 1-3 days/week) and then eat freely on nonrestricted days (Jahromi et al., 2014).
A better understanding of the role of the gut microbiota in persons with MS may lead to the development of targeted and individualized nursing interventions. Education for both the nurse and the patient on the importance of healthy diet, exercise, and the possible interaction of gut microbiota on the MS process is important during clinical encounters. The goal is that affecting the gut microbiota in patients with MS could lead to prevention, or at least a reduction, in disease progression and symptom amelioration. The use of dietary interventions in combination with improved self-care management strategies to enhance medication and diet adherence may be key to enhancing wellness in this patient population. Education on dietary self-management should be included in each encounter. Supplementation with prebiotics may be useful in maintaining microbial diversity (Scott, Gratz, Sheridan, Flint, & Duncan, 2013). Lin, Chou, Chien, Chang, & Lin (2016) found that the administration of a prebiotic/probiotic prevented the development of EAE. It remains uncertain whether this would be the case in humans and warrants additional study.
Implications for Research
Future studies on diet and nutrition and their role in MS pathophysiology and disease exacerbation are needed. In addition, studies have shown that probiotic treatment improves microbiota in rodent models (Takata et al., 2015). Research from animal models and humans supports the beneficial effects of intermittent calorie restriction on multiple outcomes, including increased survival and reduced dysbiosis. Our preliminary results are consistent with previous results (Jahromi et al., 2014). Another future direction may be the investigation of disease-modifying medications on microbiota. Because some studies have found changes in the microbiota with GA treatment, more studies are needed to test the efficacy of disease-modifying medications and vitamin D3 on disease progression. In addition, in the investigative stages is FMT for people with MS and other chronic diseases (Bakken et al., 2011).
Conclusions
The mechanism(s) by which gut microbiota may be associated with MS and its etiology and symptoms is a promising area for neuroscience practice and research. A growing body of research supports the associations between the gut microbiome the “gut-brain” connection and MS in rodent studies. However, further study is needed in humans. There are new avenues for the development of novel therapeutic and nursing intervention targets. Manipulation of the gut microbiome may be achieved by selective antibiotic treatment, the administration of immune-modifying probiotics, inexpensive fecal transplant (Borody et al., 2013), or other effective and inexpensive dietary interventions (Scott et al., 2013). The identification of appropriate microbiota and the development of proper microbiome-based treatment strategy may assist in altering the chronic disease course and prevent acute exacerbations of MS (Brody).
REFERENCES
Bakken J. S., Borody T., Brandt L. J., Brill J. V., Demarco D. C., Franzos M. A., … Fecal Microbiota Transplantation Workgroup. (2011). Treating Clostridium difficile infection with fecal microbiota transplantation. Clinical Gastroenterology and Hepatology, 9(12), 1044–1049. doi:10.1026/j.cgh.2011.08.014
Berer K., Krishnamoorthy G. (2012). Commensal gut flora and brain autoimmunity: A love or hate affair? Acta Neuropathologica, 123(5), 639–651. doi:10.1007/s00401-012-0949-9
Borody T. J., Paramsothy S., Agrawal G. (2013). Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Current Gastroenterology Reports, 15(8), 337. doi:10.1007/s11894-013-0337-1
Borody T. J., Leis S. M., Campbell J. L., Torres M., Nowak A. (2012). Could multiple sclerosis be caused by bacteria? Presented at the American College of Gastroenterology, Annual Scientific Meeting and Postgraduate Course 2011, Washington, DC.
Cantarel B. L., Waubant E., Chehoud C., Kuczynski J., DeSantis T. Z., Warrington J., Mowry E. M. (2015). Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. Journal of Investigative Medicine, 63(5), 729–734. doi:10.1097/JIM.0000000000000192
Ezendam J., de Klerk A., Gremmer E. R., van Loveren H. (2008). Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clinical and Experimental Immunology, 154(3), 424–431. doi:10.1111/j.1365-2249.2008.03788.x
Foster J. A., McVey Neufeld K. A. (2013). Gut-brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences, 36(5), 305–312. doi:10.1016/j.tins.2013.01.005
Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature, 486(7402), 207–214. doi:10.1038/nature11234
Jahromi S. R., Sahraian M. A., Ashtari F., Ayromlou H., Etemadifar M., Ghaffarpour M., Ziaie S. (2014). Islamic fasting and multiple sclerosis. BMC Neurology, 14, 56. doi:10.1186/1471-2377-14-56
Jhangi S., Ghandhi R., Glanz B., Cook S., Nejad P., Ward D., Weiner H. (2014). Increased Archaea species and changes with therapy in gut microbiome of multiple sclerosis subjects. Neurology, 82(10 Suppl.), S24.
Joscelyn J., Kasper L. H. (2014). Digesting the emerging role for the gut microbiome in central nervous system demyelination. Multiple Sclerosis, 20(12), 1553–1559. doi:10.1177/1352458514541579
Lee Y. K., Menezes J. S., Umesaki Y., Mazmanian S. K. (2011). Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl. 1), 4615–4622. doi:10.1073/pnas.1000082107
Lin S. H., Chou L. M., Chien Y. W., Chang J. S., Lin C. I. (2016). Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterology Research and Practice. Advance online publication. doi: 10.1155/2016/5789232.
Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Yamamura T. (2015). Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One, 10(9), e0137429. doi:101371/journal.pone.0137429
National MS Society. (n.d.). The basic facts. Retrieved from http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-Genetics%E2%80%94The-Basic-Facts.pdf
Newland P. K., Fearing A., Riley M., Neath A. (2012). Symptom clusters in women with relapsing-remitting multiple sclerosis. Journal of Neuroscience Nursing, 44(2), 66–71.
Ochoa-Repáraz J., Mielcarz D. W., Begum-Haque S., Kasper L. H. (2011). Gut, bugs, and brain: Role of commensal bacteria in the control of central nervous system disease. Annals of Neurology, 69(2), 240–247. doi:10.1002/ana.22344
Ochoa-Repáraz J., Mielcarz D. W., Ditrio L. E., Burroughs A. R., Begum-Haque S., Dasgupta S., Kasper L. H. (2010). Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. Journal of Immunology, 185(7), 4101–4108. doi:10.4049/jimmunol.1001443
Ochoa-Repáraz J., Mielcarz D. W., Ditrio L. E., Burroughs A. R., Foureau D. M., Haque-Begum S., Kasper L. H. (2009). Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. Journal of Immunology, 183(10), 6041–6050. doi:10.4049/jimmunol.0900747
Ochoa-Repáraz J., Riccardi C., Rynda A., Jun S., Callis G., Pascual D. W. (2007). Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. Journal of Immunology, 178(3), 1791–1799.
Riccio P., Rossano R. (2015). Nutrition facts in multiple sclerosis. American Society for Neurochemistry Neuro, 7(1). doi:10.1177/1759091414568185
Rumah K. R., Linden J., Fischetti V. A., Vartanian T. (2013). Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One, 8(10), e76359. doi:10.1371/journal.pone.0076359
Scott K. P., Gratz S. W., Sheridan P. O., Flint H. J., Duncan S. H. (2013). The influence of diet on the gut microbiota. Pharmacological Research, 69(1), 52–60. doi:10.1016/j.phrs.2012.10.020
Sommer F., Bäckhed F. (2013). The gut microbiota—Masters of host development and physiology. Nature Reviews. Microbiology, 11(4), 227–238. doi:10.1038/nrmicro2974
Takata K., Kinoshita M., Okuno T., Moriya M., Kohda T., Honorat J. A., Nakatsuji Y. (2011). The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PLoS One, 6(11), e27644. doi:10.1371/journal.pone.0027644
Takata K., Tomita T., Okuno T., Kinoshita M., Koda T., Honorat J. A., Nakatsuji Y. (2015). Dietary yeasts reduce inflammation in central nerve system via microflora. Annals of Clinical and Translational Neurology, 2(1), 56–66. doi:10.1002/acn3.153
Tremlett H., Zhao Y., Rieckmann P., Hutchinson M. (2010). New perspectives in the natural history of multiple sclerosis. Neurology, 74(24), 2004–2015. doi:10.1212/WNL.0b013e3181e3973f.
von Geldern G., Mowry E. M. (2012). The influence of nutritional factors on the prognosis of multiple sclerosis. Nature Reviews. Neurology, 8(12), 678–689. doi:10.1038/nrneurol.2012.194
Wang Y., Begum-Haque S., Telesford K. M., Ochoa-Repáraz J., Christy M., Kasper E. J., Kasper L. H. (2014). A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes, 5(4), 552–561. doi:10.4161/gmic.29797
Yang L., Nossa C. W., Pei Z. (2014). Microbial dysbiosis and esophageal diseases. In K. E. Nelson (Ed.), Encyclopedia of metagenomics. New York, NY: Springer.