Neonatal vitamin D
Why is this important to me?
A growing body of evidence suggests people with low vitamin D levels may have an increased risk of MS, just as supplementary vitamin D may have a protective effect. Exposure to the sun is one of the most important ways in which our bodies produce Vitamin D. In general, evidence suggests that people born in April and May in the Northern Hemisphere may be at increased risk of MS. Thus, these individuals went through a critical phase of their fetal development during the time of the year when individuals have the least exposure to sunlight. This study examined the relationship between vitamin D levels in newborn babies and the risk of developing MS later in life.
What is the objective of this study?
The authors of this study compared vitamin D levels in the blood of newborns who developed MS later in life to the levels in newborns who did not develop the disease.
Results showed:
- Consistent with other studies, vitamin D levels varied according to the season, with the lowest vitamin D levels in blood samples from babies born in the winter and early spring.
- Lower levels of vitamin D in newborns were associated with an increased risk of MS later in life.
- When the researchers divided the levels of vitamin D into five groups, babies with the highest level of vitamin D were 53% less likely to develop MS later in life than babies with the lowest level of vitamin D.
Previous studies had suggested a protective role for supplemental vitamin D, and the results of this study were consistent with that observation. Insufficient levels of vitamin D in the fetus may increase risk of developing MS later in life, and the study results suggest that vitamin D supplements during pregnancy may be beneficial. However, around 40% of babies in this study had low levels of vitamin D and not all went on to develop MS. Thus, other factors clearly play a role in development of MS. How vitamin D provides protection is not known precisely, but it may reduce inflammation and, in doing so, protect the brain.
This study has some limitations:
- How well newborn vitamin D levels represent levels during pregnancy is not clear.
- It is not clear at what stage of life (fetus, newborn, adolescent, etc.) that vitamin D levels are most important in protecting against MS. Larger studies that assess vitamin D levels throughout life will help clarify this question.
- This study did not investigate the impact of behaviors, such as sun exposure, outside exercise, diet, and vitamin D supplementation, during childhood or adolescence on the risk of MS.
- The oldest blood samples were collected in 1981, and the study was performed in 2012. Thus, the oldest study subjects were 31 years old, and some of these individuals may have developed MS at an older age.
- Other factors that may also influence MS risk, such as smoking, obesity, and physical activity, were not examined. Thus, vitamin D is likely one of several factors that appears to influence your risk of developing MS.
How did the authors study this issue?
Since 1981, blood has been routinely obtained from Danish newborns by heel stick 5-7 days after birth and stored in a bank called the Danish Newborn Screening Biobank. All individuals residing in Denmark with MS are registered in a database called the Danish Multiple Sclerosis Registry. Due to the use of a personal ID number assigned to each Danish citizen, the Biobank and the MS Registry are linked. The authors identified all people in Denmark who had been diagnosed with MS in 2012 or earlier. They then retrieved the blood samples for these individuals that had been stored in the Biobank. For each person with MS, they also retrieved blood samples from 1-2 people of the same sex born on the same day who had not developed MS to serve as matched controls. All blood samples were tested for levels of vitamin D. The authors examined blood samples from 521 newborns who developed MS later in life and 972 newborns who did not.
| SHARE: | |||||
Original Article
Neonatal vitamin D status and risk of multiple sclerosis
Neurology
Nete Munk Nielsen, MD, MSc, PhD*; Kassandra L. Munger, MSc, ScD*; Nils Koch-Henriksen, MD, DMSc; David M. Hougaard, MD, DMSc; Melinda Magyari, MD, PhD; Kristian T. Jørgensen, MSc, PhD; Marika Lundqvist, MSc; Jacob Simonsen, MSc, PhD; Tine Jess, MD, DMSc; Arieh Cohen, MSc, PhD; Egon Stenager, MD, DMSc‡; Alberto Ascherio, MD, DrPH‡
Objective: As previous research has suggested that exposure to vitamin D insufficiency in utero may have relevance for the risk of multiple sclerosis (MS), we aimed to examine the direct association between level of neonatal vitamin D and risk of MS.
Methods: We carried out a matched case-control study. Dried blood spots samples (DBSS) belonging to 521 patients with MS were identified in the Danish Newborn Screening Biobank. For every patient with MS, 1–2 controls with the same sex and birth date were retrieved from the Biobank (n 5 972). Level of 25-hydroxyvitamin D (25[OH]D) in the DBSS was measured using liquid chromatography tandem mass spectroscopy. The association between different levels of 25(OH)D and risk of MS was evaluated by odds ratios (OR) calculated in conditional logistic regression models.
Results: We observed that lower levels of 25(OH)D in neonates were associated with an increased risk of MS. In the analysis by quintiles, MS risk was highest among individuals in the bottom quintile (,20.7 nmol/L) and lowest among those in the top quintile of 25(OH)D ($48.9 nmol/L), with an OR for top vs bottom of 0.53 (95% confidence interval [CI] 0.36–0.78). In the analysis treating 25(OH)D as a continuous variable, a 25 nmol/L increase in neonatal 25(OH)D resulted in a 30% reduced risk of MS (OR 0.70, 95% CI 0.57–0.84).
Conclusion: Low concentrations of neonatal vitamin D are associated with an increased risk of MS. In light of the high prevalence of vitamin D insufficiency among pregnant women, our observation may have importance for public health. Neurology® 2017;88:1–8
GLOSSARY
25(OH)D = 25-hydroxyvitamin D; 25(OH)D2 = 25-hydroxyvitamin D2; 25(OH)D3 = 25-hydroxyvitamin D3; CI = confidence interval; DBSS = dried blood spots samples; DNSB = Danish Newborn Screening Biobank; MS = multiple sclerosis; OR = odds ratio
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS affecting approximately 2.5 million individuals worldwide.1,2 The etiology and exact pathogenesis of MS is still unknown, but there is growing evidence that low vitamin D levels are associated with an increased risk of MS3,4 and that supplementary vitamin D intake may have a protective effect.5,6 The results of a recent mendelian randomization study strongly support the causality of an association between lower vitamin D levels and increased susceptibility to MS.7
Although some controversy exists, patients with MS from the Northern hemisphere seem to be more likely to be born in April or May,8–12 just after the very low winter levels of vitamin D,13 suggesting that in utero exposure to vitamin D insufficiency also is relevant for the risk of MS.14
In a Swedish case-control study,15 no association was found between levels of neonatal 25-hydroxyvitamin D (25[OH]D) measured in dried blood spots samples (DBSS) and later risk of MS, but the results may have been affected by degradation of 25(OH)D due to high storage temperatures.15,16 In contrast, in a recent study in the Finnish Maternity Cohort, maternal vitamin D deficiency (serum 25[OH]D levels ,30 nmol/L) in early pregnancy was associated with a 90% increase in risk of MS in the offspring.17
Here, we examine the association between neonatal 25(OH)D status and risk of MS in a large population-based case-control study using data from the nationwide Danish MS registry and the Danish Newborn Screening Biobank (DNSB).
METHODS
Participants. The study was based on a linkage between the Danish Multiple Sclerosis Registry,18 the Danish Civil Registration System,19 and DBSS from the DNSB.20 Residual DBSS have been systematically stored at 2208 C for individuals born in Denmark since May 1, 1981. The biobank is considered close to complete for virtually all newborns in Denmark since 1982, corresponding to approximately 1.8 million samples. DBSS are taken by a heel prick 5–7 days after birth and analyzed for a number of inborn errors of metabolism at the Newborn Screening Laboratory, Statens Serum Institut, in Copenhagen.20,21 Using data from the nationwide Danish Multiple Sclerosis Registry (MS Registry), we identified all the individuals who were born since April 30, 1981, and had onset of MS before or in 2012. The Danish MS Registry, which was established in 1956, is the longest running nationwide MS registry and covers a period of more than 50 years.18 In the present study, we only included cases fulfilling the diagnostic criteria of Allison or Poser including possible MS (when other diseases are ruled out), and since 2005 the McDonald criteria and their updates, but not cases of clinically isolated syndrome or poorly documented cases. The linkage between the MS Registry and the DNSB was possible due to the unique personal ID number assigned to every Danish citizen by the Civil Registration System since April 1968.19 For every MS case with a DBSS in the DNSB, DBSS from 1 to 2 controls of same sex and exact birth date were selected. Controls had to be alive or living in Denmark on the date of MS diagnosis in the matched patient with MS.
25(OH)D assessment. We measured 25-hydroxyvitamin D3 (25[OH]D3), which is the main circulating form of vitamin D,22 and the related ergosterol-derived form 25-hydroxyvitamin D2 (25[OH]D2), which can be obtained from certain dietary sources, e.g., mushrooms, fortified foods, and vitamin supplements.21 For each individual, one 3.2-mm disc of DBSS was used. The assay was performed at the Statens Serum Institut, Copenhagen, and is a modified version of the method by Eyles et al.23 The assay method is highly sensitive and uses minimal sample cleanup to reduce sample loss during extraction, chemical derivatization to enhance 25(OH)D2 and 25(OH)D3 ionization, and liquid chromatography tandem mass spectroscopy coupled with multiple reaction monitoring. It has previously been shown that the assay can reliably detect seasonal (within year) fluctuations, is strongly correlated with neonatal cord blood (r 5 0.85),13 and that appropriate 25(OH)D3 concentrations can be detected after prolonged storage time (e.g., greater than 20 years).23 Neonatal DBSS are routinely collected from capillary blood. The protein-bound 25(OH)D is excluded from erythrocytes, thus, concentrations in whole blood are lower than in serum. We therefore multiplied the total 25(OH)D concentrations measured in capillary blood by 2.56 (1/[1– 0.61]) to estimate serum levels13,23; all 25(OH)D values reported below correspond to estimated serum levels.
Potetial confounders. . Perinatal factors such as gestational age, birthweight, maternal age at birth, Apgar score, and parental ethnicity have been shown to be associated with neonatal vitamin D status or maternal D vitamin deficiency,24,25 whereas the association with MS is less clear.26–28 Data on birthweight (,3,000, 3,000–3,249, 3,250–3,499, 3,500–3,749, 3,750–3,999, 4,000– 4,249, $4,250 g), Apgar score 5 minutes after birth (0–9, 10), and gestational age (#37, 38, 39, 40, $41 weeks) were obtained from the Danish Medical Birth Register.29 Information on parents’ age at child’s birth (,20, 20–24, 25–29, 30–34, 35–39, $40 years) and place of birth (both parents Danish born, both born abroad or one born abroad and one unknown/with missing information, one Danish and one born abroad, one Danish born and one unknown/missing information) was obtained from the civil registration system.19 Greenland was considered a foreign country. Parental place of birth was used as a proxy measure of ethnicity. Parental history of MS was determined using information from the MS Registry.
Statistics. Odds ratios (ORs) of MS according to neonatal levels of 25(OH)D were estimated using conditional logistic regression. Due to the matching scheme, all analyses were controlled for sex, age, and exact date of birth. To explore a dose-response relationship with MS, we modeled neonatal 25(OH)D levels in 5 groups according to quintiles of 25(OH)D in controls. Individuals were furthermore classified into predefined categories of 25(OH)D levels, i.e., individuals with neonatal 25 (OH)D serum levels ,30 nmol/L, 30–,50 nmol/L, and $50 nmol/L were considered to have been born with deficient, insufficient, and sufficient levels, respectively, of 25(OH)D.22,25 Under an assumption of linearity between levels of 25(OH)D and MS risk, we finally modeled 25(OH)D as a continuous variable. We a priori included gestational age, birthweight, and parental ethnicity in the multivariable conditional logistic regression analysis. Other covariates (mother’s age, father’s age, Apgar score, and parental MS) were only retained in the model if they changed at least one of the quintile-specific ORs estimated in the above multivariable model by $10%. In the multivariable analysis, we excluded cases and controls having missing values.
Standard protocol approvals, registrations, and patient consents. The present study was approved by the Danish Ethical Committee of the Capital Region, Copenhagen, and The Danish Data Protection Agency.
RESULTS
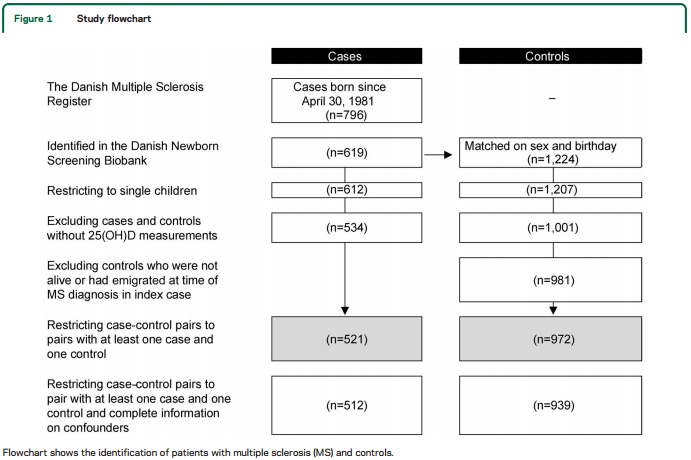
According to the MS Registry, 796 individuals born on or after April 30, 1981, had developed MS. One hundred seventy-seven patients with MS, 68% of whom were born before 1986, were not identified in DNSB. Restricting to singletons and excluding individuals who did not have sufficient DBSS to measure 25(OH)D, the number of cases and controls was reduced to 534 and 1,001. Twenty of the controls died or had emigrated from Denmark before MS diagnosis in the corresponding case. Finally, we excluded 13 cases without a matched control, and 9 controls without a case, thus limiting the dataset to 521 cases and 972 controls (figure 1).
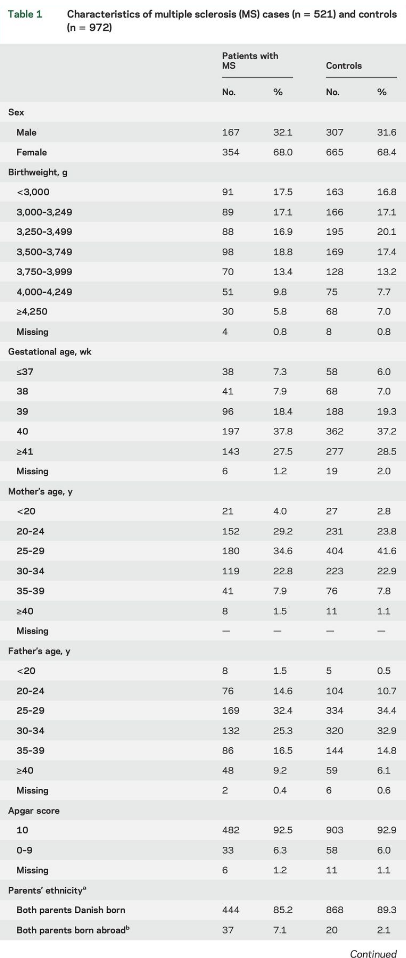
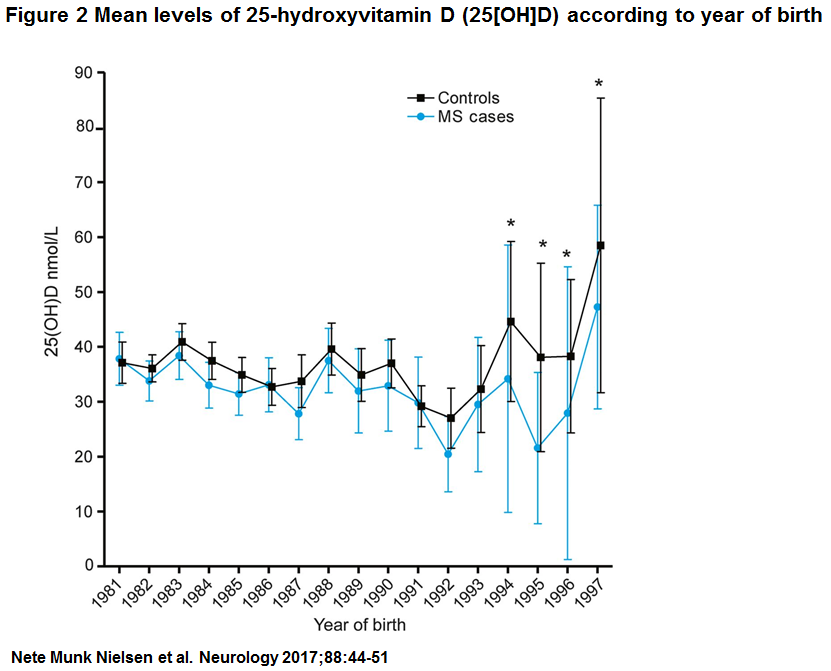
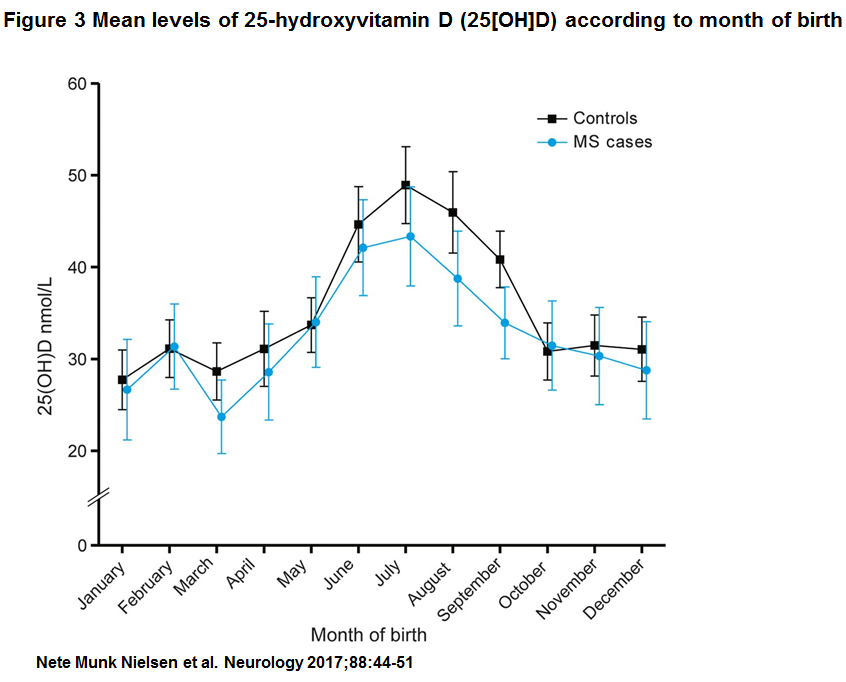
Characteristics of patients with MS and controls participating in the study are described in table 1. Mean values of serum 25(OH)D were 33.0 nmol/L (SD 16.9, minimum 1.3 nmol/L, maximum 101.4 nmol/L) among patients with MS and 35.9 nmol (SD 17.5, minimum 4.9, maximum 114.7) among controls (p , 0.01) (figure e-1 at Neurology.org). Importantly, 25(OH)D levels measured in DBSS from persons born in the period 1981 to 2000 showed no evidence of 25(OH)D degradation by year for cases or controls (figure 2). Figure 3 shows mean 25 (OH)D levels according to month of birth among cases and controls. As expected, a clear seasonal variation was seen for cases as well as for controls, with the lowest levels of 25(OH)D in the winter and early spring.
We observed that lower levels of neonatal 25(OH) D were associated with an increased risk of MS. In the analyses by quintiles, MS risk decreased monotonically with increasing 25(OH)D (OR for the highest compared to lowest quintile 0.53, 95% confidence interval [CI] 0.36–0.78; p for trend 0.001, table 2).
A similar pattern was seen using predefined categories of 25(OH)D, although no statistically significant difference was observed between sufficient and insufficient levels of 25(OH)D (table 2). Finally, for every 25 nmol/L increase in neonatal 25(OH)D level, the risk of MS was reduced by 30% (OR 0.70, 95% CI 0.57–0.84) (table 2).
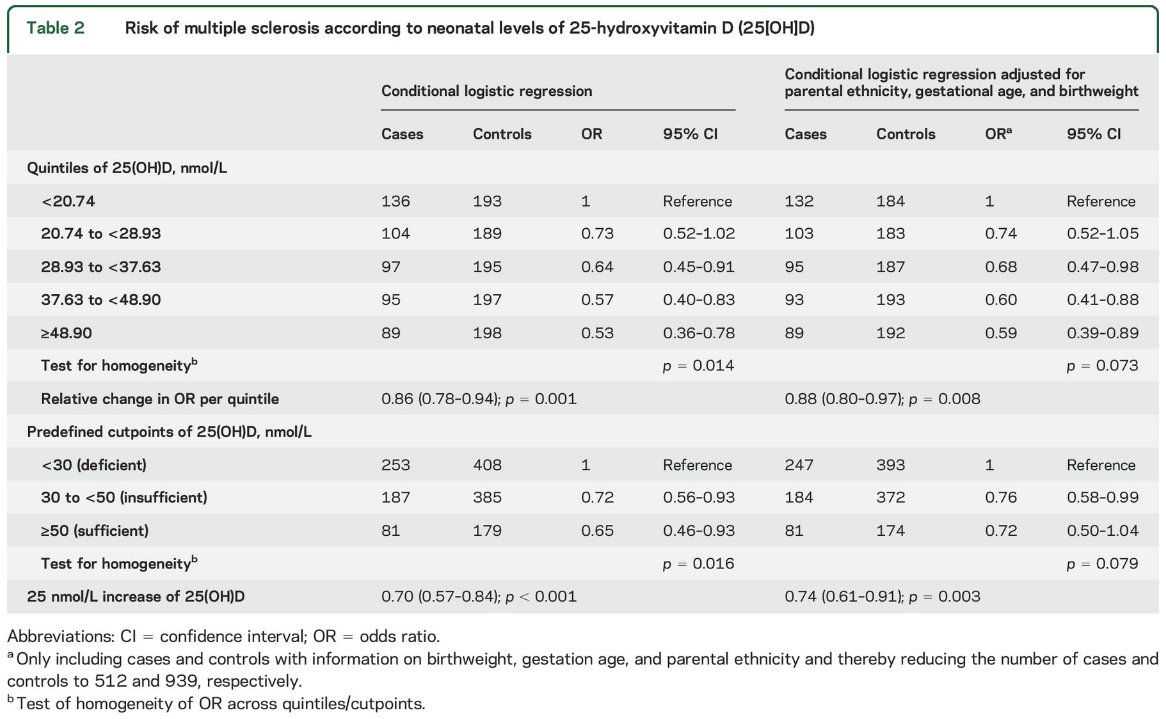
In the multivariable analyses, we ended up adjusting only for parental ethnicity, birthweight, and gestational age, and results were practically unchanged (table 2).
DISCUSSION
In this large population-based casecontrol study, we found an inverse association between neonatal levels of 25(OH)D and risk of MS. Especially children born with 25(OH)D levels less than 30 nmol/L were at an increased risk of MS, whereas the additional benefit of higher levels of 25(OH)D were less strong. Our results support previous studies suggesting a protective role of vitamin D in the development of MS, but also suggests that vitamin D insufficiency in utero may be of relevance for the risk of MS.
The majority of previous studies have examined the relevance of vitamin D intake, UV radiation, or serum 25(OH)D levels in adolescence or adulthood4,6,16,30–32; few studies have examined the possible association between in utero vitamin D levels and risk of MS, and findings are conflicting.3,14,15,17 Furthermore, direct measurements of in utero levels of D vitamin are not possible, meaning that the above association has only been addressed using different markers of fetal vitamin D levels, including maternal intake of vitamin D14 during pregnancy, maternal serum levels of 25(OH)D during pregnancy,3,17 or neonatal levels of vitamin D.15
A study among US nurses found a lower MS risk in the offspring of women with higher vitamin D intake during pregnancy.14 Results should, however, be interpreted with caution because food intake during pregnancy relied on self-reported data collected several decades after the pregnancy at a point when many patients with MS already had been diagnosed among the offspring, which increases the risk of recall bias.
Two prospective, nested case-control studies have been conducted measuring maternal serum 25(OH) D in pregnancy (primarily 1st trimester) and risk of MS in the offspring. In the first of these studies, conducted in Sweden and including 37 patients with MS and 185 matched controls, there was no significant association between serum 25(OH)D levels in pregnancy and MS risk among the offspring.3 This analysis was, however, limited by the small sample size and by insufficient matching on date of birth. More recently, a larger study was conducted in the Finnish Maternity Cohort. In this study, which included 176 patients with MS and 326 matched controls, maternal vitamin D deficiency during pregnancy (25[OH] D , 30 nmol/L) was associated with a 90% increased risk of MS in the offspring.17
Finally, in a Swedish case-control study, neonatal levels of 25(OH)D were measured in DBSS stored in the Swedish Phenylketonuria Biobank since 1975. In this study, similar in design to ours and including 459 cases and 663 controls, levels of neonatal 25 (OH)D were not associated with MS risk.15 Several limitations of the study may have contributed to the null findings, including low participation among controls (;44%) as compared to cases (;88%), so that the representativeness of the controls is uncertain.16 In addition, Ueda and colleagues15 reported a substantial degradation of 25(OH)D in older DBSS, which is likely to be due to storage conditions. DBSS in the Swedish Phenylketonuria Biobank were stored at room temperature from 1975 to August 1981, and since then at 48 C. Approximately 50% of the DBSS were collected between 1975 and 1981 and thus exposed to room temperature. In our study, there was no evidence of 25(OH)D degradation, likely due to storage of the DBSS at 2208 C.20 Degradation is expected to be similar among cases and controls, and is likely to result in nondifferential misclassification,16 which could also explain the null finding in the Swedish study. Ueda and colleagues15 did repeat the analyses stratifying on 5-year interval of birth year, and interestingly, they observed a tendency towards a lower risk of MS the higher the level of neonatal 25(OH)D levels for individuals born between 1980 and 1984. Thus, for every 10 nmol/L increase in neonatal 25(OH)D level, the risk of MS was reduced by 10% (OR 0.9 [0.78–1.01]), which is consistent with our findings.
A potential limitation of our study is that only 534 of the 796 patients with MS born since the establishment of the DNSB were included in the 25 (OH)D analysis, mainly due to lack of DBSS material or due to less completeness of the DNSB in the first years after establishment. However, inadequate material is unlikely to be related to 25(OH)D levels or future MS risk and thus inconsequential for the results of the study. Further, 25(OH)D levels in the present study were low, with more than 40% of the children born with deficient levels of 25(OH)D (,30 nmol/L). Nevertheless, our measured levels of neonatal 25(OH)D are compatible with neonatal values measured in a previous study by Eyles et al.13 Another important issue is how well neonatal 25 (OH)D levels measured in DBSS represent 25(OH) D status during pregnancy. 25(OH)D levels in DBSS are highly correlated with levels in umbilical cord blood,13 but probably only moderately correlated with maternal levels at midgestation.25 Thus in a recent longitudinal study in the Netherlands, the correlation between maternal serum levels of 25(OH) D at midgestation and levels of 25(OH)D in the offspring’s umbilical cord was 0.57 and levels of 25(OH) D in umbilical cord were about 20 nmol/L lower than maternal serum levels at midgestation.25
Data concerning sun exposure, including outdoor physical activity, body mass index, diet, and intake of vitamin D supplements in adolescence and adult life were not available for cases and controls in our study, which could have influenced our results.25,33 However, in previous studies14,15 adjustment for these covariates did not change the results.
Individuals participating in the present study were young (the majority born after 1981), and many had not yet reached the age of peak MS incidence by the time of the study (2012). Thus our findings largely reflect the association between neonatal vitamin D levels and MS diagnosis up to age 30, and may not necessary be applicable to MS onset in older individuals.
A key question is whether the lower MS risk among children born with higher 25(OH)D levels reflects a direct protective effect of higher 25(OH)D levels in utero, or rather if it is mediated by higher vitamin D status during childhood and early adulthood. The association between 25(OH)D levels at birth and later in life is most likely to occur because of a combination of behavioral (similarity between mother and child in vitamin D intake and sunlight exposure) and genetic factors. Although genetic variations in 25(OH)D levels are modest34 and by themselves insufficient to explain the strong association found in this study, the impact of behavioral factors is more difficult to quantify, and we cannot therefore exclude the possibility that 25(OH)D levels in childhood and adolescence account for the apparent protective effects of in utero 25(OH)D.
Nevertheless, our results support previous suggestions of a protective role for vitamin D in the development of MS.16,32 Although the exact mechanism of a vitamin D effect is incompletely understood, it is likely to involve an attenuation of the T-cell response to autoantigens and suppression of the production of proinflammatory cytokines such as interferon-γ and Th17-interleukin 17.35 By modifying and regulating the production and release of neurotrophic factors, vitamin D may also have a neuroprotective effect.36 Animal studies have furthermore suggested that prenatal exposure to vitamin D deficiency may lead to alteration in mitochondrial, cytoskeletal, and synaptic functions of the brain.37,38
We observed an inverse association between neonatal levels of 25(OH)D and risk of MS, thus the higher the level of 25(OH)D among neonates the lower their risk of MS in later life. However, we cannot exclude the possibility that this apparent beneficial effect is mediated by a correlation between 25(OH)D levels at birth and levels later in life, in which case maternal vitamin D supplementation would not reduce MS risk in the offspring. This uncertainty notwithstanding, the high global prevalence of hypovitaminosis D among pregnant women25,39 and the fact that increasing maternal vitamin D levels is likely to reduce the mother's risk of MS as well as her offspring's provides a rationale for universal vitamin D supplementation in pregnancy.
AUTHOR CONTRIBUTIONS
All the authors contributed to the conception and design of the study. A.A., K.L.M., N.M.N., N.K.-H., M.M., E.S., D.M.H., A.C., and M.L. contributed to the acquisition of data. A.A., K.L.M., J.S., and N.M.N. performed the statistical analyses; D.M.H., A.C., and M.L. carried out the vitamin D analyses. All the authors contributed to drafting a significant portion of the manuscript and the figures.
STUDY FUNDING
The study was supported by the Danish Society of Multiple Sclerosis and the Aase & Ejnar Danielsen's Foundation. Drs. Ascherio and Munger are supported by research grants from the US NIH (NIH R01NS073633 and R01NS046635) and National Multiple Sclerosis Society for the investigation of the role of vitamin D and other risk factors in MS.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
FOOTNOTES
↵* These authors contributed equally to this study and should be considered as co–first authors.
↵‡ These authors contributed equally to this study and should be considered as co–last authors.
Go to Neurology.org for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article.
Received April 1, 2016. Accepted in final form August 24, 2016
REFERENCES
1. Word Health Organization. Neurological Disorders: Public Health Challenges. Geneva: Word Health Organization; 2006:85–95.
2. Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532.
3. Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012;79:2140–2145.
4. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838.
5. Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65.
6. Munger KL, Chitnis T, Frazier AL, Giovannucci E, Spiegelman D, Ascherio A. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol 2011;258:479–485.
7. Mokry LE, Ross S, Ahmad OS, et al. Vitamin D and risk of multiple sclerosis: a mendelian randomization study. PLoS Med 2015;12:e1001866.
8. Bayes HK, Weir CJ, O’Leary C. Timing of birth and risk of multiple sclerosis in the Scottish population. Eur Neurol 2010;63:36–40.
9. Grytten N, Torkildsen O, Aarseth JH, et al. Month of birth as a latitude-dependent risk factor for multiple sclerosis in Norway. Mult Scler 2013;19:1028–1034.
10. Torkildsen O, Grytten N, Aarseth J, Myhr KM, Kampman MT. Month of birth as a risk factor for multiple sclerosis: an update. Acta Neurol Scand Suppl 2012:58–62.
11. Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ 2005; 330:120.
12. Templer DI, Trent NH, Spencer DA, et al. Season of birth in multiple sclerosis. Acta Neurol Scand 1992;85: 107–109. 13. Eyles DW, Morley R, Anderson C, et al. The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr Perinat Epidemiol 2010;24: 303–308.
14. Mirzaei F, Michels KB, Munger K, et al. Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol 2011;70:30–40.
15. Ueda P, Rafatnia F, Baarnhielm M, et al. Neonatal vitamin D status and risk of multiple sclerosis. Ann Neurol 2014;76:338–346.
16. Ascherio A, Munger KL. Not too late to take vitamin D supplements. Ann Neurol 2014;76:321–322.
17. Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel HM, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol 2016;73:515–519.
18. Bronnum-Hansen H, Koch-Henriksen N, Stenager E. The Danish Multiple Sclerosis Registry. Scand J Public Health 2011;39:62–64.
19. Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 2006;53:441–449.
20. Norgaard-Pedersen B, Hougaard DM. Storage policies and use of the Danish Newborn Screening Biobank. J Inherit Metab Dis 2007;30:530–536.
21. McGrath JJ, Eyles DW, Pedersen CB, et al. Neonatal vitamin D status and risk of schizophrenia: a populationbased case-control study. Arch Gen Psychiatry 2010;67: 889–894.
22. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281.
23. Eyles D, Anderson C, Ko P, et al. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin Chim Acta 2009;403:145–151.
24. Sablok A, Batra A, Thariani K, et al. Supplementation of vitamin D in pregnancy and its correlation with fetomaternal outcome. Clin Endocrinol 2015;83:536–541.
25. Vinkhuyzen AA, Eyles DW, Burne TH, et al. Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: the Generation R Study. J Steroid Biochem Mol Biol Epub 2015 Sep 15.
26. Gardener H, Munger KL, Chitnis T, Michels KB, Spiegelman D, Ascherio A. Prenatal and perinatal factors and risk of multiple sclerosis. Epidemiology 2009;20:611–618.
27. Montgomery SM, Lambe M, Olsson T, Ekbom A. Parental age, family size, and risk of multiple sclerosis. Epidemiology 2004;15:717–723.
28. Mueller BA, Nelson JL, Newcomb PA. Intrauterine environment and multiple sclerosis: a population-based casecontrol study. Mult Scler 2013;19:106–111.
29. Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320–323.
30. Lucas RM, Ponsonby AL, Dear K, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011;76:540–548.
31. Mesliniene S, Ramrattan L, Giddings S, Sheikh-Ali M. Role of vitamin D in the onset, progression, and severity of multiple sclerosis. Endocr Pract 2013;19:129–136.
32. Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010;9:599–612.
33. Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N. Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One 2011;6:e22179.
34. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–188.
35. Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 2013;45:217–226.
36. Wrzosek M, Lukaszkiewicz J, Wrzosek M, et al. Vitamin D and the central nervous system. Pharmacol Rep 2013; 65:271–278.
37. Eyles D, Almeras L, Benech P, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007;103: 538–545.
38. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 2007;7:769–780.
39. Saraf R, Morton SM, Camargo CA Jr, Grant CC. Global summary of maternal and newborn vitamin D status: a systematic review. Matern Child Nutr 2016;12:647–668.
