Impact of Delayed Diagnosis and Treatment in CIS
Why is this important to me?
A single episode of one or more neurological symptoms caused by inflammation or loss of myelin (the fatty substance that wraps around and protects nerve fibers) in the brain is called “clinically isolated syndrome” or CIS. If you have CIS, you may experience vision problems, spasticity, or muscle and sensation problems. CIS is often a precursor to MS. Around 56-88% of patients with CIS who also have brain abnormalities on an MRI will be diagnosed with MS within 5-14 years. This study examined whether there is benefit to starting disease-modifying therapies in people with CIS in order to achieve better outcomes instead of waiting for diagnosis of MS.
What is the objective of this study?
The author reviewed information from clinical trials and other sources about the impact of treatment and the importance of diagnosis in people with CIS.
The data that the author reviewed suggest that the treatment of people with CIS with the disease-modifying treatment:
- Appears to reduce the occurrence of converting from CIS to MS
- Seemed to reduce the number of new, active lesions in the brain.
- Appears to reduce disability progression.
- May have lead to a smaller decrease in brain volume.
- Appeared to lower the probability and delay the conversion from CIS to MS
Whether you have CIS or MS, you should watch for any new or recurring symptom that could indicate a change in your neurological status. Always report a new symptom or one that occurs with increasing frequency to your healthcare provider. Early treatment has been shown to help reduce rate of relapse, slow progression and disability, and delay the conversion from CIS to MS. to MS. Sharing information with your healthcare provider is the best way to ensure that you are receiving safe and effective disease-modifying therapy.
How did the author study this issue?
The author reviewed results from clinical trials and other published information about the importance of early diagnosis and treatment of CIS and MS.
| SHARE: | |||||
Original Article
Impact of Delayed Diagnosis and Treatment in Clinically Isolated Syndrome and Multiple Sclerosis
Journal of Neuroscience Nursing
Patricia M. Kennedy
Multiple sclerosis (MS) is a progressive inflammatory disease with several possible clinical courses; before the development of definite MS, some patients may have clinically isolated syndrome(CIS), which is a single attack of neurological symptoms caused by inflammation or demyelination. Disease-modifying treatments (DMTs) have been extensively used for the management of MS, resulting in improvements in the clinical presentation and decreases in MS-associated neurological damage. Earlier initiation of DMT in the course of MS is associated with better outcomes. For patients with CIS, initiation of interferon-beta or glatiramer acetate treatment after an initial clinical event indicative of MS has been associated with delays in the progression to clinically definite MS as well as improvements in measures of neurological damage via magnetic resonance imaging. The initiation of treatment for patients with CIS should be considered, and nurses play a vital role in educating patients about the risks of conversion to MS and the benefits of early DMT.
Multiple sclerosis (MS) is a progressive inflammatory disease affecting the white matter of the brain and spinal cord (Compston & Coles, 2002; Gold, Wolinsky, Amato, & Comi, 2010). The primary pathological process involved in the development and progression of MS is the immune-mediated destruction of the myelin sheath, resulting in damage to or loss of the axons (Calabresi, 2004; Chiaravalloti & DeLuca, 2008). The MS is characterized by the development of sclerotic plaques, or lesions, in different areas of the central nervous system (CNS) that are associated with demyelination of neurons (Compston & Coles, 2002; Gold et al., 2010). Magnetic resonance imaging (MRI) is one of the most useful diagnostic tools for evaluating the presence, number, and location of MS lesions; all three of these parameters are used for the diagnosis of MS and for assessing disease progression (Calabresi, 2004). The dissemination of these lesions in both space (i.e., occurring in different areas of the brain and spine) and time (i.e., new lesions observed at follow-up) represents a major diagnostic criterion for MS (Calabresi, 2004; Polman et al., 2011). An MS lesion appears as an area of high signal on T2-weighted images; the presence of a new T2-hyperintense lesion (a high-signal-intensity lesion on a T2-weighted image) detected 30 or more days after a first clinical MS event can be indicative of the dissemination of lesions in time. Gadolinium (Gd)-enhanced T1-weighted MRI can be used to differentiate between active and inactive lesions; a Gd-enhanced lesion appears on the MRI as a bright spot and is a site of ongoing inflammation or an active lesion (Charil & Filippi, 2007; Sicotte, 2011). This demyelination of the axons that accompanies the formation of MS lesions results in impaired conduction of action potentials, causing many of the signs and symptoms of MS (Compston & Coles, 2002), including vision impairment, paresthesia, weakness, fatigue, depression, numbness, cognitive dysfunction, bladder dysfunction, dizziness, spasticity, and balance or coordination impairment (Calabresi, 2004; Chiaravalloti & DeLuca, 2008).
There are four main subtypes of MS that differ in the clinical course of disease progression: relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), progressive-relapsing MS, and primary-progressive MS (PPMS; Gold et al., 2010; Miller, Barkhif, Montalban, Thompson, & Filippi, 2005). The most commonly diagnosed type of MS is RRMS, with approximately 85% of all new cases of MS diagnosed as RRMS (Gold et al., 2010; Hawker, 2011; Hurwitz, 2009; Miller et al., 2005). Patients with RRMS experience recurrent attacks (or relapses) of neurological symptoms, followed by complete or partial remissions of symptoms (Gold et al., 2010; Miller et al., 2005). These relapses are associated with acute, inflammatory MS lesions that occur because of a local increase in the permeability of the blood brain barrier and immune cell infiltration; the subsequent remission of symptoms is related to the resolution of inflammation and some remyelination (Charil & Filippi, 2007). Patients with RRMS may also experience persistent disability related to the accumulation of irreversible neurological damage (Charil & Filippi, 2007; Hurwitz, 2009). A subset of patients with RRMS have benign MS, experiencing few MS symptoms, and remain highly functional; however, only approximately half of patients diagnosed with benign MS at 10 years after the initial MS presentation continue to experience a benign disease course (Hurwitz, 2009). Approximately 90% of patients with untreated RRMS progress to SPMS within 25 years (Gold et al., 2010; Hurwitz, 2009). Patients with SPMS generally experience relatively steady progression of disability, with a decrease in or absence of the distinct attacks that characterize RRMS (Gold et al., 2010; Hurwitz, 2009; Miller et al., 2005). Some patients with SPMS, however, may experience periods of disease stability or intermittent relapses (Hawker, 2011; Hurwitz, 2009). One of the rarest subtypes of MS, progressive-relapsing MS, is diagnosed in approximately 1% of patients with MS and is characterized by ongoing progression from disease onset, with one or more severe symptom attacks occurring with continuing progression (Gold et al., 2010; Hawker, 2011). PPMS is less frequently observed (occurring in approximately 10% of patients diagnosed with MS) and is characterized by steady progression of disability from onset with no discrete relapses or remissions (Gold et al., 2010).
In addition to the four major clinical subtypes of MS, patients may experience a single attack of one or more neurological symptoms; such a unifocal or multifocal episode is known as clinically isolated syndrome (CIS) and is caused by inflammation or demyelination at one or more locations in the CNS (Gold et al., 2010; Ross & Thrower, 2010). Patients with CIS commonly present with optic neuritis, long-tract symptoms or signs, brainstem syndrome, or multifocal symptoms (Confavreux et al., 2000; Miller et al., 2005; Pelidou, Giannopoulos, Tzavidi, Lagos, & Kyritsis, 2008). For patients with CIS, the presence of one or more MRI lesions indicative of demyelination is associated with a higher risk of experiencing a second attack and developing clinically definite MS (CDMS; Gold et al., 2010; Miller et al., 2005; Ross & Thrower, 2010). In previous studies of patients with CIS, the rate of conversion to CDMS ranged from 56% to 88% for patients with an abnormal MRI during mean follow-up periods ranging from 5 to 14 years; in contrast, the rate of conversion to CDMS was approximately 8%–24% for patients with a normal MRI (Coyle, 2008). Early diagnosis of CIS, particularly CIS with an abnormal MRI, will allow for earlier monitoring and treatment of patients with MS, resulting in improved patient outcomes.
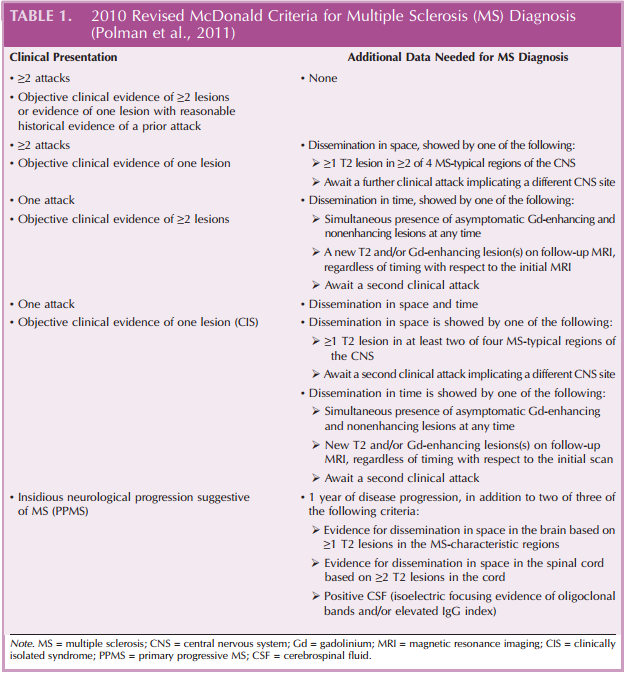
Along with differential diagnosis, MS is primarily diagnosed based on the presentation of symptoms suggestive of demyelination and the dissemination of MS lesions (as detected by MRI) in time and space (Polman et al., 2011). The McDonald Criteria of the International Panel on Diagnosis of MS outline the clinical, laboratory, and MRI findings that are largely used for the diagnosis of MS (Table 1). The use of the McDonald Criteria, which have been shown to be both sensitive and specific for the definitive diagnosis of MS, has been associated with decreases in the time to MS diagnosis (Polman et al., 2011).
Delays in the diagnosis of MS are associated with a substantially worse prognosis and disease progression. The axonal loss that is one of the hallmarks of MS pathology and is associated with cognitive impairment and disability may occur early in the course of the disease and is irreversible (Comi, 2008; Glanz, Healy, Hviid, Chitnis, & Weiner, 2012; Reuter et al., 2011; Rieckmann, 2005). Earlier diagnosis and treatment of patients with MS, including those with CIS, may reduce axonal loss and accompanying neurological symptoms (Comi, 2008; Glanz et al., 2012; Reuter et al., 2011; Rieckmann, 2005). In the previously described Canadian retrospective database analysis, longer delays in the time to referral to an MS specialist were associated with significantly worse disability, as indicated by a higher Expanded Disability Status Scale (EDSS; p < .0001). Recent studies have shown the efficacy of disease-modifying treatments (DMTs), not only for reducing the rate of relapses and slowing the course of MS progression but also for delaying the conversion from CIS to CDMS (Carter & Keating, 2010; Clerico et al., 2008a; Comi et al., 2009; Kappos et al., 2006). The benefits of using DMTs early in the course of MS, particularly for patients with CIS, and the risks associated with delayed diagnosis and treatment of MS will be reviewed in the following sections.
Benefits of Eary Treatment
The use of DMTs for the management of MS over the past approximately 20 years has resulted in substantial changes in the course of MS, reducing the MRI lesion load, reducing the relapse rate, and slowing disease and disability progression (Clerico, Rivoiro, Contessa, Viglietti, & Durelli, 2008b; Lim & Constantinescu, 2010). Recent evidence indicates that earlier initiation of DMT results in improved treatment efficacy and patient outcomes (Comi, 2008).
Interferon-Beta
The benefits of early interferon-beta (IFNB) treatment for preventing conversion from CIS to CDMS have been evaluated in a number of studies (Applebee & Panitch, 2009; Comi, 2008). The primary outcomes for the major clinical trials of IFNB in patients with CIS are summarized in Table 2 (Comi et al., 2012, 2001; Jacobs et al., 2000; Kappos et al., 2006). The Betaferon in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT) study was a 2-year, randomized, double-blind, placebo-controlled study of IFNB-1b (250 μg subcutaneously [SC] every other day) in 468 patients with CIS, as indicated by a first clinical event suggestive of MS that lasted at least 24 hours and signs and symptoms indicating the presence of at least one lesion in the CNS (Kappos et al., 2006). Patients who received IFNB-1b treatment in the BENEFIT study experienced significant delays compared with those who received placebo in the time to the development of CDMS (p < .0001) and the time to diagnosis of MS according to the McDonald criteria (p < .00001; Kappos et al., 2006). In the placebo group, 45% of patients underwent conversion to CDMS, and 85% were diagnosed with MS according to the McDonald criteria after 2 years; by contrast, only 28% of patients in the IFNB-1b group underwent conversion to CDMS, and 69% were diagnosed with MS according to the McDonald criteria after 2 years (Kappos et al., 2006). The risk of developing CDMS and McDonald MS during the 2-year BENEFIT study was approximately 50% lower in the IFNB-1b group than in the placebo group (Kappos et al., 2006). The effects of IFNB-1b treatment delays were evaluated in an open-label extension study (with all patients receiving IFNB-1b) that enrolled patients from the BENEFIT study (Kappos et al., 2007, 2009). Patients who completed the BENEFIT study and chose to enroll in the open-label extension study received IFNB-1b (250 μg SC every other day) for up to 5 years after randomization in the parent study; those patients who had received IFNB-1b during the initial 2-year double-blind BENEFIT study were considered as the early treatment group, whereas those patients who had initially received placebo were considered as the late treatment group. The risk of developing CDMS was reduced by 41% at 3 years after initial randomization in the BENEFIT study for patients in the early treatment group (37% risk of developing CDMS) compared with those in the late treatment group (51% risk of developing CDMS; hazard ratio [95% confidence interval]: 0.59 [0.44, 0.80]; Kappos et al., 2007). At 5 years after initial randomization in the BENEFIT study, a comparable reduction (37%) to that observed at 3 years in the risk of developing CDMS was observed for patients in the early treatment group versus those in the late treatment group (Kappos et al., 2009). Delays in the conversion from CIS to CDMS with IFNB-1b treatment over the 2-year BENEFIT study were observed, regardless of preexisting risk factors, including patients with multifocal CIS and subclinical disease dissemination (Polman et al., 2008).
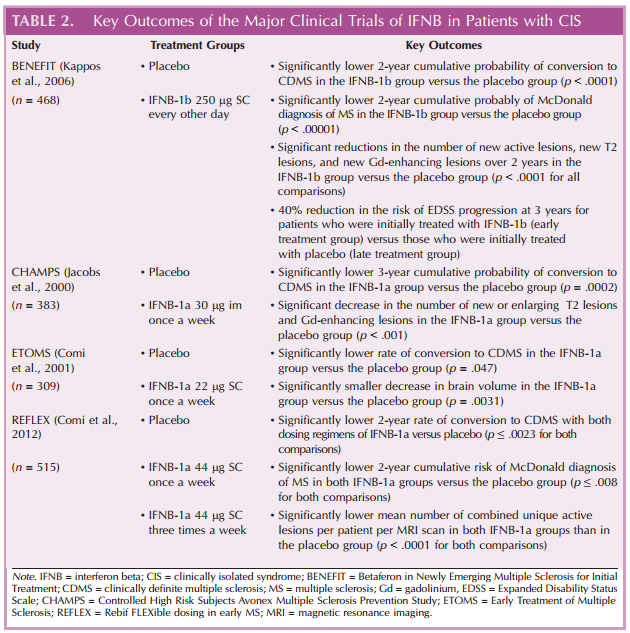
Delays in the conversion from CIS to CDMS have also been observed with IFNB-1a treatment (Comi et al., 2012; Filippi et al., 2004; Kinkel et al., 2011, 2006; Motamed, Najimi, & Fereshtehnejad, 2007; O’Connor, 2003; Pakdaman et al., 2007). The Controlled High-Risk Subjects Avonex Multiple Sclerosis Prevention Study (CHAMPS) was a 3-year, randomized, double-blind, placebo-controlled study of IFNB-1a (30 μg intramuscularly [im] once a week) in 383 patients with CIS (as indicated by a first clinical demyelinating event and at least two clinically silent MS lesions of a diameter of 3 mm or more by MRI; Jacobs et al., 2000). In CHAMPS, the cumulative 3-year probability of developing CDMS was significantly lower in the IFNB-1a group (35%) than in the placebo group (50%; p = .0002; Jacobs et al., 2000). Post hoc analyses of data from CHAMPS for patients with CIS with certain risk factors for CDMS development showed significant reductions in the risk of developing CDMS with IFNB-1a treatment compared with placebo over 3 years in patients with at least nine T2-weighted lesions (p = .0044; O’Connor et al., 2009). Similar reductions in the risk of developing CDMS with IFNB-1a treatment were observed in post hoc analyses of CHAMPS data in patients with or without Gd-enhancing lesions (p ≤ .0405 for both comparisons; O’Connor et al., 2009) and in patients with at least nine T2-weighted hyperintense lesions and at least one Gd-enhanced lesion on T1 images on the baseline MRI (p = .002; O’Connor, 2003). The effects of delays in IFNB-1a treatment were evaluated in 5- and 10-year follow-up studies to CHAMPS in which (regardless of initial assignment) all patients received IFNB-1a (30 μg im once a week; Kinkel et al., 2006, 2011). Compared with patients in the delayed treatment group (those who received placebo during the initial double-blind CHAMPS study), patients in the immediate treatment group (those who initially received IFNB-1a) had a significantly lower cumulative probability of developing CDMS at 5 years (p = .03; Kinkel et al., 2006), a significantly lower 10-year rate of CDMS (p = .004; Kinkel et al., 2011), and a significantly lower annualized relapse rate from year 5 to year 10 (p = .03; Kinkel et al., 2011).
Similar reductions in the rate of conversion from CIS to CDMS have been observed in other studies of IFNB-1a for CIS (Comi et al., 2012, 2001; Pakdaman et al., 2007) Two 2-year, randomized, double-blind placebo-controlled studies evaluated the different doses of IFNB-1a in patients with CIS: the Early Treatment of Multiple Sclerosis Study (ETOMS; 22 μg SC once a week; Comi et al., 2001) and the Rebif FLEXible dosing in early MS (REFLEX) study (44 μg SC three times a week or once a week; Comi et al., 2012). In ETOMS, the rate of conversion to CDMS was significantly lower for patients in the IFNB-1a group (34%) than for those in the placebo group (45%; p = .047; Comi et al., 2001). In the REFLEX study, the 2-year rate of conversion to CDMS was significantly lower for both dosing regimens of IFNB-1a (21% for three times a week and 21% for once a week) compared with placebo (37.5%; p ≤ .0023 for both comparisons; Comi et al., 2012). The 2-year cumulative risk of converting to McDonald-diagnosed MS was also significantly lower for patients receiving both doses of IFNB-1a in the REFLEX study compared with those receiving placebo (p ≤ .0080 for both comparisons; Comi et al., 2012). A meta-analysis of data from two studies of IFNB-1a (CHAMPS and ETOMS) and one study of IFNB-1b (BENEFIT) for CIS supported the results of the individual studies; the risk of conversion from CIS to CDMS was reduced by 51% with IFNB treatment versus placebo, and treatment with IFNB delayed the conversion from CIS to CDMS from 317 days to 363 days (Melo, Rodrigues, & Bar-Or, 2008).
In addition to slowing conversion from CIS to CDMS, treatment with IFNB for patients with CIS has been associated with significant improvements in MRI measures of MS (Filippi et al., 2004; Jacobs et al., 2000; Kappos et al., 2006). In the BENEFIT study, significant reductions were observed in the cumulative numbers of new active lesions, new T2 lesions, and new Gd-enhancing lesions over 2 years for patients who received IFNB-1b compared with those who received placebo (p < .0001 for all comparisons; Kappos et al., 2006). In CHAMPS, the increase in the volume of T2 brain lesions, number of new or enlarging T2 lesions, and number of Gd-enhancing lesions at 18 months were all significantly lower for patients in the IFNB-1a group than for those in the placebo group (p < .001 for all comparisons; Jacobs et al., 2000). In an analysis of MRI data from the ETOMS study, the reduction in brain volume at 24 months after the start of treatment was significantly lower for patients who received IFNB-1a than for those who received placebo (p = .0031; Filippi et al., 2004). In the REFLEX study, the mean number of combined unique active lesions per patient per MRI scan was significantly lower in both IFNB-1a groups than in the placebo group (p < .0001 for both comparisons; Comi et al., 2012).
IFNB treatment has also been shown to result in reductions in the number of relapses and to slow the progression of disability in patients with CIS (Motamed et al., 2007; Trojano et al., 2009). The risk of progression of disability on the EDSS was reduced by 40% at 3 years after initial randomization in the BENEFIT study for patients in the early treatment group (those who were initially randomized to IFNB-1b) compared with those in the late treatment group (those who were initially randomized to placebo; Kappos et al., 2007). In a randomized, placebo-controlled study of IFNB-1a for CIS, the mean number of new relapses and changes in the EDSS were significantly lower in the IFNB-1a group than in the untreated group (p ≤ .034 for both comparisons; Motamed et al., 2007).
Although early treatment with IFNB results in improved patient outcomes, IFNB is associated with adverse events (AEs) that must be taken into consideration when initiating treatment. Among the most common AEs associated with IFNB treatment are flu-like symptoms (including myalgia, headache, fever, and fatigue), gastrointestinal upset (including abdominal pain and nausea), elevation of liver enzymes, depression, hematological abnormalities, and injection site reactions (Avonex, 2006; Betaseron, 2008; Rebif, 2008). Other serious AEs that have been associated with IFNB treatment include suicidal ideation, anaphylaxis, and seizure (Avonex, 2006; Betaseron, 2008; Rebif, 2008). IFNB-1a and IFNB-1b are considered Pregnancy Category C agents (Avonex, 2006; Betaseron, 2008; Rebif, 2008).
Glatiramer Acetate
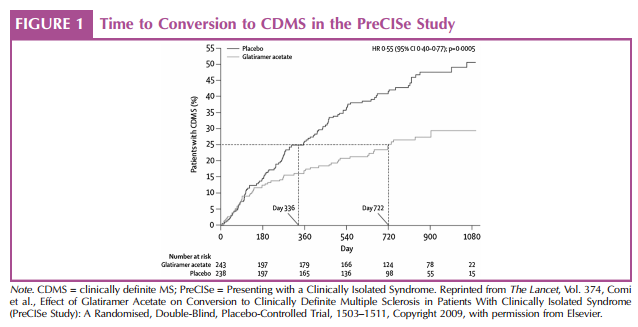
The efficacy of glatiramer acetate treatment for patients with CIS has been evaluated in a randomized, double-blind study, the PreCISe (early glatiramer acetate treatment in delaying conversion to CDMS in subjects Presenting with a Clinically Isolated Syndrome) study (Comi et al., 2009). In that study, 481 patients with one unifocal demyelinating event and at least two 6-mm or larger T2 cerebral lesions received glatiramer acetate (20 mg SC daily) or placebo until they converted to CDMS or for up to 36 months (Comi et al., 2009). Patients in the glatiramer acetate group had a significant 45% lower risk of conversion to CDMS than patients in the placebo group (p = .0005). Conversion from CIS to CDMS was significantly delayed by 386 days in the glatiramer acetate group compared with the placebo group (p = .0041; Figure 1; Comi et al., 2009). The percentage of patients who experienced a second attack in the glatiramer acetate group (24.7%) was significantly lower than the percentage of patients who experienced a second attack in the placebo group (p < .0001). Patients who received glatiramer acetate also developed significantly fewer new T2 lesions by months 12 and 24 and at the end of treatment than patients who received placebo (p < .0001 for all comparisons; Comi et al., 2009). Significant reductions were also observed in the cumulative number of T2 lesions and Gd-enhancing lesions by the end of treatment (p < .0001 for both comparisons; Comi et al., 2009).
Glatiramer acetate, like IFNB, has a generally favorable safety and tolerability profile (Galetta & Markowitz, 2005; Weber et al., 2012). The most common AEs associated with glatiramer acetate treatment include injection site reactions, vasodilatation, chest pain, asthenia, infection, pain, nausea, arthralgia, anxiety, and hypertonia (Copaxone, 2007). Patients treated with glatiramer acetate may also experience an immediate postinjection reaction, with symptoms such as flushing, chest pain, palpitations, anxiety, dyspnea, constriction of the throat, and urticaria; these symptoms are generally short lived and resolve without treatment (Copaxone, 2007). Glatiramer acetate is considered a Pregnancy Category B agent (Copaxone, 2007).
Risks of Delayed MS Diagnosis and Treatment
As described in the previous section, there is a growing body of evidence that early treatment of CIS with disease-modifying therapeutics results in improved clinical outcomes (Comi et al., 2012, 2009; Kappos et al., 2009, 2006; Kinkel et al., 2011; Motamed et al., 2007; O’Connor et al., 2009; Polman et al., 2008). There are certain MRI results and clinical features in patients with CIS that are associated with a worse MS prognosis, including brain atrophy, T2 lesion load, number of Gd-enhancing lesions, and lower number of focal systems involved in the initial demyelinating event (Di et al., 2010). Brain atrophy, which is indicative of the irreversible axonal damage that accompanies MS and leads to neurological disability, can be detected early in the course of MS (Di et al., 2010; Gold et al., 2010; Goodin & Bates, 2009). In a recent study of 99 patients with CIS over 6 years (Di et al., 2010), the mean annual brain atrophy rate was −0.38% overall and was significantly higher for patients who had CDMS at 6 years (−0.50%) compared with patients who did not progress to CDMS at 6 years (−0.26%; p = .035). In the same study of 99 patients with CIS (Di et al., 2010), patients with a higher T2 lesion volume at baseline were at a higher risk of progressing CDMS; higher T2 lesion load and number of Gd-enhancing lesions were also associated with higher levels of disability at 6 years, based on the EDSS.
The MS is associated with negative effects on many different aspects of cognitive functioning, including long-term memory, efficacy of information processing, speed of processing, executive functions, and visual perceptual functions (Chiaravalloti & DeLuca, 2008). Recent studies have also shown that cognitive impairment develops early in the course of MS and is associated with higher lesion loads (Glanz et al., 2012; Reuter et al., 2011). In a study of 90 patients with a diagnosis of CIS or CDMS and a disease duration of no more than 6 years since the initial symptom (Glanz et al., 2012), a significant decline was observed in a measure of the speed of information processing and working memory and in measures of immediate and delayed visual spatial memory (p ≤ .0009 for all comparisons). A separate study of 24 patients with CIS and a high risk of developing MS showed similar results; 29% of patients had cognitive impairment at baseline, whereas 54% had cognitive impairment at 5 years follow-up (Reuter et al., 2011). Patients with a higher number of T2 lesions on MRI at baseline had a higher cognitive impairment at 5 years follow-up (Reuter et al., 2011). In a study of 62 patients with CIS (Summers et al., 2008), the presence of Gd-enhancing lesions at baseline and new T2 lesions after 3 months were associated with the development of cognitive impairment.
Patient-specific factors and clinical disease features may also be predictive of the development of CDMS in patients with CIS. In a study of 330 patients with CIS, non-White race/ethnicity and younger age were both significantly associated with an increased risk of a second demyelinating event within a year (p < .0001 for both comparisons; Mowry et al., 2009). Patients with a lower number of functional systems affected by the first demyelinating event were also at a higher risk for a second demyelinating event (p = .011; Mowry et al., 2009).
Even beyond the first demyelinating event of MS, there are detriments associated with delays in the diagnosis and treatment of MS (Putzki et al., 2009; Salter, Cutter, Tyry, Marrie, & Volmer, 2010; Scalfari et al., 2010; Scott & Schramke, 2010). In a study of 1,157 patients with untreated RRMS, quality of life at baseline was significantly lower for patients with MS compared with healthy controls; initiation of IFNB-1a (30 μg im once a week) treatment resulted in an improvement in measures of health-related quality of life for patients with MS (Putzki et al., 2009). EuroQol 5-Dimension utility and visual analog scale scores improved significantly from baseline at 12 months of treatment with IFNB-1a (p ≤ .0046 for both comparisons). IFNB-1a therapy was also associated with a significant decrease from baseline in the mean annual relapse rate at 12 months (p < .0001). A separate study showed that a higher number of relapses and shorter intervals between relapses were risk factors for the development of more severe neurodegeneration and disability in patients with RRMS (Scalfari et al., 2010).
Delays in the diagnosis and treatment of MS, which may be associated with a number of factors, allow for the accumulation of axonal damage, causing more severe neurological disability and the progression of MS (Fernandez et al., 2010; Gold et al., 2010; Goodin & Bates, 2009; Gout et al., 2011; Kelly et al., 2011; Kingwell et al., 2010; Rieckmann, 2005). In a study of 147 Spanish patients diagnosed with MS, the median time from the onset of symptoms to the first consultation with a physician was 19.2 months, whereas the median time between the initial consultation and confirmed diagnosis of MS by a specialized neurological unit was 5.7 months, resulting in a median overall time from symptom onset to diagnosis of approximately 2 years (Fernandez et al., 2010). A retrospective analysis of referral and diagnostic delay data from two Canadian databases showed that younger age at onset of MS symptoms was associated with a significantly longer delay in diagnosis of MS and referral to an MS specialist (p < .001); significantly longer delays in diagnosis and referral were also observed for patients with PPMS compared with those with relapsing MS at onset (p < .001; Kingwell et al., 2010). Of particular concern has been the observation that many of the patients presenting with CIS have in fact experienced previous events consistent with demyelination (Fernandez et al., 2010; Gout et al., 2011). In the previously described study in Spanish patients, extensive interviews conducted at specialized MS units showed that approximately a quarter of patients who were referred with a diagnosis of CIS had experienced more than one prior attack (indicating CDMS; Fernandez et al., 2010). A separate study of 178 patients consulting for CIS showed that 44% of patients reported prior symptoms indicative of a demyelinating event on a self-administered questionnaire; the presence of these events was validated by a neurologist for 70% of these patients (Gout et al., 2011).
Role of the Nurse in the Early Diagnosis and Treatment of MS
As described in the previous section, there are some MRI parameters, patient-specific factors, and clinical features that are associated with worse outcomes for patients with MS. Early evaluation of these factors may assist in identifying patients who would most benefit from disease-modifying therapeutics at early stages of MS, particularly patients with CIS (Goodin et al., 2011; Lebrun et al., 2008; Lukas et al., 2010; Summers et al., 2008; Thrower, 2007; Tintore et al., 2006). The course of disease monitoring and treatment will largely depend on the presence or absence of these risk factors as well as the type of MS (Ross & Thrower, 2010; Thrower, 2007; Webb, 2008). Patients with radiologically isolated syndrome, or MS lesions by MRI but no clinical symptoms, require careful monitoring and should be closely followed by a neurologist, with a particular view of identifying early clinical signs of MS or dissemination of MS lesions in time and space by MRI (Ross & Thrower, 2010). Patients with MS symptoms may or may not have lesions on MRI assessments; those patients with one or more MS lesions detected by MRI at the time of presentation for CIS have an approximately two-fold higher risk of developing CDMS than patients with a normal MRI (Thrower, 2007). Patients with MS lesions detected by MRI should be advised of the associated risk of developing CDMS. Regardless of whether patients are diagnosed with CIS or CDMS, patients should receive information and education regarding coping with the diagnosis and treatment options to assist in alleviating some of the stress associated with receiving a diagnosis of MS (Ross & Thrower, 2010). The nurse may play a critical role in assisting patients with handling their diagnosis and understanding their options for disease treatment and management (Ross & Thrower, 2010). Nurses may also play a vital role in assisting patients with finding outside support (e.g., counselors) to assist them in coping with their MS (Webb, 2008).
Addressing the factors associated with delays in diagnosis may allow for earlier recognition and treatment of MS. For those patients initially identified as having CIS but who decline treatment, nurses can provide in-depth education related to the risks of waiting to initiate treatment (Ross & Thrower, 2010; Webb, 2008). In addition, they can advise their patients to watch for symptoms that indicate a change in their neurological status and a possible progression from CIS to CDMS and can stress the importance of being seen by an MS specialist quickly (Ross & Thrower, 2010).
In addition, discussions about early treatment and its benefits should also include information about AEs that patients might experience with treatment. When considering IFNB treatment, patients should be made aware of the potential for developing flu-like symptoms, depression, injection site reactions, elevated liver enzyme levels, and hematological abnormalities (Avonex, 2006; Betaseron, 2008; Rebif, 2008). With glatiramer acetate, patients should be alerted to the potential for injection-related skin disruptions, which may be problematic and increase the risk of nonadherence, and postinjection reactions, which are physiologically benign but can be psychologically frightening (Copaxone, 2007). Patients should also be advised regarding the assessment and management of these AEs. For example, patients receiving IFNB should be monitored for drug effects, blood cell counts, and liver function by undergoing regular complete blood and differential white blood cell counts and platelet counts and blood chemistries, including liver function tests (Avonex, 2006; Betaseron, 2008; Rebif, 2008). Patients could also be instructed on self-care for other side effects, including flu-like symptoms or injection site reactions.
Early initiation of DMT has been associated with significant improvements in MS prognosis (Applebee & Panitch, 2009; Carter & Keating, 2010; Comi, 2008). A consensus statement by the National Multiple Sclerosis Society regarding the use of DMTs in MS stressed the importance of making these treatments available early in the course of disease progression, highlighting recent clinical results, which showed better outcomes with earlier initiation of therapy (National Pharmaceutical Council Inc., 2005). One of the primary barriers to the widespread use of DMT early in the course of MS, particularly for patients with CIS, may be patient resistance to initiating treatment before developing “true” MS. For example, despite the positive results obtained in the BENEFIT study, the perceptions of IFNB-1b treatment and the desire to continue with treatment varied from patient to patient (Webb, 2008). Among patients who did not experience new MS symptoms, some patients reported relief that the IFNB-1b therapy appeared to be working, whereas others remained unconvinced of the value of continued treatment (particularly those patients who experienced side effects, such as flu-like symptoms; Webb, 2008). The decision of whether to initiate DMT when patients present with CIS should be the result of a shared decision making between the patient and the provider; however, nurses can provide invaluable support to patients considering DMT early in the MS disease course (Webb, 2008). Nurses can play a major role in the education of patients regarding the risk of developing CDMS for patients with CIS and the benefits of DMT for improving MS prognosis (Ross & Thrower, 2010; Webb, 2008). Nurses with a working knowledge of the most current DMTs may be better able to provide up-to-date information about the benefits and disadvantages of these treatments (Ross & Thrower, 2010). Patients need to be made aware of the fact that neurological damage can occur very early in the disease process (Webb, 2008). Nurses also need to emphasize the irreversible nature of MS-induced neurological damage and the clinical consequences of that damage (Webb, 2008). The greater success of DMT earlier in the clinical course of MS should be emphasized as well as the potential of DMT to prevent MS-related neurological damage (Ross & Thrower, 2010; Webb, 2008). A discussion of the potential side effects associated with DMT, along with the need for long-term, continuous therapy for treatment success, may assist the patient in making an informed decision regarding the early treatment of MS (Ross & Thrower, 2010; Webb, 2008). To assist patients in adhering to DMT over the long term, nurses can educate patients regarding the self-administration of treatment and strategies to reduce DMT-related side effects (Webb, 2008).
Conclusions
Delays in the diagnosis and treatment of MS may result from a number of factors, including delays in the time to initial consultation and delays in the time to referral to a specialists, and are generally associated with poorer outcomes (Fernandez et al., 2010; Gout et al., 2011; Kelly et al., 2011; Kingwell et al., 2010). Early treatment of CIS with DMTs is associated with delays in the development of CDMS and slowing of the progression of MS-related disability and the development of brain and spinal cord lesions (Comi et al., 2012, 2009; Kappos et al., 2009, 2006; Kinkel et al., 2011; Motamed et al., 2007; O’Connor et al., 2009; Polman et al., 2008; Ross & Thrower, 2010). Nurses play a vital role in increasing the efficiency of MS diagnosis and in informing patients of the numerous benefits of early initiation of DMT for MS (Ross & Thrower, 2010; Webb, 2008).
References
Applebee A., Panitch H. (2009). Early stage and long term treatment of multiple sclerosis with interferon-beta. Biologics, 3, 257–271.
Avonex. (2006). Avonex (Interferon beta-1a) IM Injection [package insert]. Cambridge, MA: Biogen Idec.
Betaseron. (2008). Betaseron (Interferon beta-1b) for SC Injection [package insert]. Montville, NJ: Bayer HealthCare Pharmaceuticals.
Calabresi P. A. (2004). Diagnosis and management of multiple sclerosis. American Family Physician, 70, 1935–1944.
Carter N. J., Keating G. M. (2010). Glatiramer acetate: A review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs, 70, 1545–1577.
Charil A., Filippi M. (2007). Inflammatory demyelination and neurodegeneration in early multiple sclerosis. Journal of the Neurological Sciences, 259, 7–15.
Chiaravalloti N. D., DeLuca J. (2008). Cognitive impairment in multiple sclerosis. Lancet Neurology, 7, 1139–1151.
Clerico M., Faggiano F., Palace J., Rice G., Tintore M., Durelli L. (2008a). Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis. Cochrane Database of Systemic Reviews, (2), CD005278.
Clerico M., Rivoiro C., Contessa G., Viglietti D., Durelli L. (2008b). The therapy of multiple sclerosis with immune-modulating or immunosuppressive drug. A critical evaluation based upon evidence based parameters and published systematic reviews. Clinical Neurology and Neurosurgery, 110, 878–885.
Comi G. (2008). Clinically isolated syndrome: The rationale for early treatment. Nature Clinical Practice Neurology, 4, 234–235.
Comi G., De S. N., Freedman M. S., Barkhof F., Polman C. H., Uitdehaag B. M., Kappos L. (2012). Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): A phase 3 randomised controlled trial. Lancet Neurology, 11, 33–41.
Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernandez O., Hommes O. R. (2001). Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet, 357, 1576–1582.
Comi G., Martinelli V., Rodegher M., Moiola L., Bajenaru O., Carra A., Filippi M. (2009). Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): A randomised, double-blind, placebo-controlled trial. Lancet, 374, 1503–1511.
Compston A., Coles A. (2002). Multiple sclerosis. Lancet, 359, 1221–1231.
Confavreux C., Vukusic S., Moreau T., Adeleine P. (2000). Relapses and progression of disability in multiple sclerosis. New England Journal of Medicine, 343, 1430–1438.
Copaxone. (2007). Copaxone (glatiramer acetate injection) [package insert]. Kansas City, MO: TEVA Neuroscience.
Coyle P. K. (2008). Early treatment of multiple sclerosis to prevent neurologic damage. Neurology, 71, S3–S7.
Di F. M., Anderson V. M., Altmann D. R., Swanton J. K., Plant G. T., Thompson A. J., Miller D. H. (2010). Brain atrophy and lesion load measures over 1 year relate to clinical status after 6 years in patients with clinically isolated syndromes. Journal of Neurology, Neurosurgery and Psychiatry, 81, 204–208.
Fernandez O., Fernandez V., Arbizu T., Izquierdo G., Bosca I., Arroyo R., de R. E. (2010). Characteristics of multiple sclerosis at onset and delay of diagnosis and treatment in Spain (the Novo Study). Journal of Neurology, 257, 1500–1507.
Filippi M., Rovaris M., Inglese M., Barkhof F., De S. N., Smith S., Comi G. (2004). Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet, 364, 1489–1496.
Galetta S. L., Markowitz C. (2005). US FDA-approved disease-modifying treatments for multiple sclerosis: Review of adverse effect profiles. CNS Drugs, 19, 239–252.
Glanz B. I., Healy B. C., Hviid L. E., Chitnis T., Weiner H. L. (2012). Cognitive deterioration in patients with early multiplesclerosis: A 5-year study. Journal of Neurology, Neurosurgery and Psychiatry, 83, 38–43.
Gold R., Wolinsky J. S., Amato M. P., Comi G. (2010). Evolving expectations around early management of multiple sclerosis. Therapeutic Advances in Neurological Disorders, 3, 351–367.
Goodin D. S., Bates D. (2009). Treatment of early multiple sclerosis: the value of treatment initiation after a first clinical episode. Multiple Sclerosis, 15, 1175–1182.
Goodin D. S., Traboulsee A., Knappertz V., Reder A. T., Li D., Langdon D., Ebers G. C. (2011). Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon beta-1b trial in multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry, 83, 282–287.
Gout O., Lebrun-Frenay C., Labauge P., Le Page G. E., Clavelou P., Allouche S. (2011). Prior suggestive symptoms in one-third of patients consulting for a “first” demyelinating event. Journal of Neurology, Neurosurgery and Psychiatry, 82, 323–325.
Hawker K. (2011). Progressive multiple sclerosis: Characteristics and management. Neurologic Clinics, 29, 423–434.
Hurwitz B. J. (2009). The diagnosis of multiple sclerosis and the clinical subtypes. Annals of Indian Academy of Neurology, 12, 226–230.
Jacobs L. D., Beck R. W., Simon J. H., Kinkel R. P., Brownscheidle C. M., Murray T. J., Sandrock A. W.; the CHAMPS Study Group (2000). Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. New England Journal of Medicine, 343, 898–904.
Kappos L., Freedman M. S., Polman C. H., Edan G., Hartung H. P., Miller D. H., Sandbrink R. (2007). Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: A 3-year follow-up analysis of the BENEFIT study. Lancet, 370, 389–397.
Kappos L., Freedman M. S., Polman C. H., Edan G., Hartung H. P., Miller D. H., Pohl C. (2009). Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurology, 8, 987–997.
Kappos L., Polman C. H., Freedman M. S., Edan G., Hartung H. P., Miller D. H., Sandbrink R. (2006). Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology, 67, 1242–1249.
Kelly S. B., Chaila E., Kinsella K., Duggan M., McGuigan C., Tubridy N., Hutchinson M. (2011). Multiple sclerosis, from referral to confirmed diagnosis: An audit of clinical practice. Multiple Sclerosis, 17, 1017–1021.
Kingwell E., Leung A. L., Roger E., Duquette P., Rieckmann P., Tremlett H. (2010). Factors associated with delay to medical recognition in two Canadian multiple sclerosis cohorts. Journal of the Neurological Sciences, 292, 57–62.
Kinkel R. P., Dontchev M., Kollman C., Skaramagas T. T., O’Connor P. W., Simon J. H. (2011). Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: A 10-year follow-up of the controlled high-risk Avonex multiple sclerosis prevention study in ongoing neurological surveillance. Archives of Neurology, 69, 183–190.
Kinkel R. P., Kollman C., O’Connor P., Murray T. J., Simon J., Arnold D., Wall M.CHAMPIONS study Group. (2006). IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology, 66, 678–684.
Lebrun C., Bensa C., Debouverie M., de S. J., Wiertlievski S., Brochet B., Roullet E. (2008). Unexpected multiple sclerosis: Follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. Journal of Neurology, Neurosurgery and Psychiatry, 79, 195–198.
Lim S. Y., Constantinescu C. S. (2010). Current and future disease-modifying therapies in multiple sclerosis. International Journal of Clinical Practice, 64, 637–650.
Lukas C., Minneboo A., de Groot V., Moraal B., Knol D. L., Polman C. H., Vrenken H. (2010). Early central atrophy rate predicts 5 year clinical outcome in multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry, 81, 1351–1356.
Melo A., Rodrigues B., Bar-Or A. (2008). Beta interferons in clinically isolated syndromes: A meta-analysis. Arquivos de Neuro-Psiquiatria, 66, 8–10.
Miller D., Barkhof F., Montalban X., Thompson A., Filippi M. (2005). Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurology, 4, 281–288.
Motamed M. R., Najimi N., Fereshtehnejad S. M. (2007). The effect of interferon-beta1a on relapses and progression of disability in patients with clinically isolated syndromes (CIS) suggestive of multiple sclerosis. Clinical Neurology and Neurosurgery, 109, 344–349.
Mowry E. M., Pesic M., Grimes B., Deen S. R., Bacchetti P., Waubant E. (2009). Clinical predictors of early second event in patients with clinically isolated syndrome. Journal of Neurology, 256, 1061–1066.
National Pharmaceutical Council Inc. (2005). Pain: Current understanding of assessment, management, and treatments. Retrieved from www.npcnow.org/resources/PDFs/PainAddendum.pdf
O’Connor P. (2003). The effects of intramuscular interferon beta-Ia in patients at high risk for development of multiple sclerosis: A post hoc analysis of data from CHAMPS. Clinical Therapeutics, 25, 2865–2874.
O’Connor P., Kinkel R. P., Kremenchutzky M. (2009). Efficacy of intramuscular interferon beta-1a in patients with clinically isolated syndrome: Analysis of subgroups based on new risk criteria. Multiple Sclerosis, 15, 728–734.
Pakdaman H., Sahraian M. A., Fallah A., Pakdaman R., Ghareghozli K., Ghafarpour M., Shirani A. (2007). Effect of early interferon beta-1a therapy on conversion to multiple sclerosis in Iranian patients with a first demyelinating event. Acta Neurologica Scandinavica, 115, 429–431.
Pelidou S. H., Giannopoulos S., Tzavidi S., Lagos G., Kyritsis A. P. (2008). Multiple sclerosis presented as clinically isolated syndrome: The need for early diagnosis and treatment. Therapeutics and Clinical Risk Management, 4, 627–630.
Polman C., Kappos L., Freedman M. S., Edan G., Hartung H. P., Miller D. H., Sandbrink R. (2008). Subgroups of the BENEFIT study: Risk of developing MS and treatment effect of interferon beta-1b. Journal of Neurology, 255, 480–487.
Polman C. H., Reingold S. C., Banwell B., Clanet M., Cohen J. A., Filippi M., Wolinsky J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69, 292–302.
Putzki N., Fischer J., Gottwald K., Reifschneider G., Ries S., Siever A., Hartung H. P. (2009). Quality of life in 1000 patients with early relapsing-remitting multiple sclerosis. European Journal of Neurology, 16, 713–720.
Rebif. (2008). Rebif (interferon beta-1a) sc injection [package insert]. Rockland, MA: EMD Serono.
Reuter F., Zaaraoui W., Crespy L., Faivre A., Rico A., Malikova I., Audoin B. (2011). Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry, 82, 1157–1159.
Rieckmann P. (2005). Neurodegeneration and clinical relevance for early treatment in multiple sclerosis. International MS Journal, 12, 42–51.
Ross A. P., Thrower B. W. (2010). Recent developments in the early diagnosis and management of multiple sclerosis. Journal of Neuroscience Nursing, 42, 342–353.
Salter A. R., Cutter G. R., Tyry T., Marrie R. A., Vollmer T. (2010). Impact of loss of mobility on instrumental activities of daily living and socioeconomic status in patients with MS. Current Medical Research and Opinion, 26, 493–500.
Scalfari A., Neuhaus A., Degenhardt A., Rice G. P., Muraro P. A., Daumer M., Ebers G. C. (2010). The natural history of multiple sclerosis: A geographically based study 10: Relapses and long-term disability. Brain, 133, 1914–1929.
Scott T. F., Schramke C. J. (2010). Poor recovery after the first two attacks of multiple sclerosis is associated with poor outcome five years later. Journal of the Neurological Sciences, 292, 52–56.
Sicotte N. L. (2011). Magnetic resonance imaging in multiple sclerosis: The role of conventional imaging. Neurologic Clinics, 29, 343–356.
Summers M., Swanton J., Fernando K., Dalton C., Miller D. H., Cipolotti L., Ron M. A. (2008). Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. Journal of Neurology, Neurosurgery and Psychiatry, 79, 955–958.
Thrower B. W. (2007). Clinically isolated syndromes: Predicting and delaying multiple sclerosis. Neurology, 68, S12–S15.
Tintore M., Rovira A., Rio J., Nos C., Grive E., Tellez N., Montalban X. (2006). Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology, 67, 968–972.
Trojano M., Pellegrini F., Paolicelli D., Fuiani A., Zimatore G. B., Tortorella C., Amato M. P.; Italian Multiple Sclerosis Database Network (MSDN) Group. (2009). Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Annals of Neurology, 66, 513–520.
Webb U. H. (2008). Early interferon beta treatment in multiple sclerosis: Nursing care implications of the BENEFIT study. Journal of Neuroscience Nursing, 40, 356–361.
Weber M. S., Menge T., Lehmann-Horn K., Kronsbein H. C., Zettl U., Sellner J., Stuve O. (2012). Current treatment strategies for multiple sclerosis—Efficacy versus neurological adverse effects. Current Pharmaceutical Design, 18, 209–219.
