Fatigue and Fatigability in Persons With Multiple Sclerosis
Original Article
Fatigue and Fatigability in Persons With Multiple Sclerosis
Exercise and Sport Sciences Reviews
Inge Zijdewind, Roeland F. Prak, and Ria Wolkorte
INTRODUCTION
Multiple sclerosis (MS) is a chronic, immunological, and degenerative disease of the central nervous system (CNS) (3). In the early phase of MS, activated microglia attack myelin in the CNS causing axonal demyelination, which in turn reduces action potential propagation and synchronization of neuronal input to postsynaptic cells. This inflammatory response is initially followed by a period of recovery but incomplete remyelination. After several attacks and prolonged demyelination, however, the resulting cell death leads to structural changes in both white and grey matter in cortical, subcortical, and spinal levels (6). These structural changes provoke both deterioration and functionally beneficial/compensatory changes with the net effect determining the quality of the output (performance) and the effort needed for cognitive and motor function.
Clinically, disease progression typically is categorized into three phenotypes (15): 1. a primary progressing type (PPMS) in which symptoms become worse through time, 2. a relapsing-remitting type (RRMS) during which symptoms are intermitted with periods of complete or almost complete recovery, and 3. a secondary progressive stage in which persons with RRMS eventually develop symptoms without periods of recovery (secondary progressive MS, SPMS). Approximately 80% of the persons with MS begin with the RRMS phenotype and most eventually develop SPMS. The reduced capacity of the CNS to recover after several attacks most likely marks the transition of RRMS into SPMS.
Besides diminished motor, sensory, and cognitive function, individuals with MS typically report elevated levels of fatigue (12). Despite 50 yr of research on the etiology of fatigue in MS, the underlying mechanisms remain poorly understood. Part of the difficulty is the conceptualization of fatigue. In neurological patients, fatigue is characterized as a symptom and should be distinguished from fatigability, which indicates how quickly a specific level of fatigue is achieved. Current taxonomies identify two types of fatigability, one related to anticipated capabilities (perceived fatigability) and the other corresponding to use-dependent declines in performance (performance fatigability) (11). Perceived fatigability depends on the psychological state of the performer and the physiological capacity of the body to maintain homeostasis. In contrast, performance fatigability depends on the ability of the nervous system to provide an adequate activation signal for the task and the contractile capabilities of the involved muscles. Based on these distinctions, an individual could report an elevated level of fatigue due to disturbances among the factors that contribute to perceived fatigability, which could be independent of the adjustments that constrain performance fatigability. Nonetheless, there undoubtedly are significant interactions between the factors that influence the two domains of fatigability.
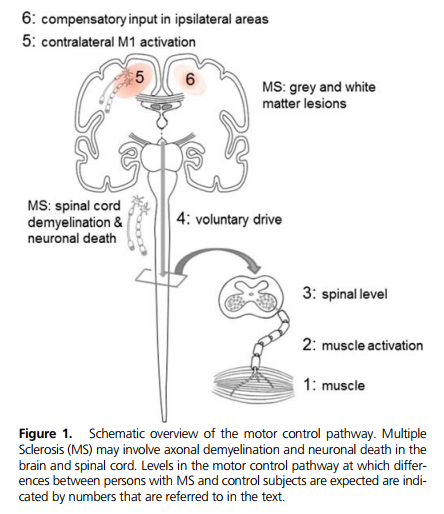
Performance fatigability can be studied during sustained or repeated motor tasks. Motor tasks have the advantage that (during maximal contractions) several steps along the output path can be monitored (Fig. 1) and the output can be measured with force transducers. Moreover, the fact that maximal efforts can be asked from subjects and evaluated makes these motor tasks more suitable to study use-dependent processes in contrast to cognitive tasks. In the next section, we discuss these use-dependent changes at different levels of the neuromuscular system but focus on those processes that likely differ between control subjects and persons with MS. We also discuss the MS-induced structural and functional changes at the cortical, spinal, and peripheral levels in relation to task performance. Most of the data discussed in the current article were obtained in persons with RRMS or SPMS. There are less data on persons with PPMS. In the second part of the article, we examine the association between the selfreported levels of fatigue and performance fatigability as indicated by the decline in force during a sustained contraction in persons with MS.
Functional Changes Due to Multiple Sclerosis The activation signals required to perform voluntary actions arises in primary and secondary motor areas in the cerebral cortex (Fig. 1, steps 5 and 6) and then projects (Fig. 1, step 4) down to the motor neurons in the spinal cord (Fig. 1, step 3). Motor neurons are the nerve cells that innervate muscle fibers and these cells are the only pathway by which the activation signal can be delivered to the muscle fibers (Fig. 1, steps 1 and 2), hence the term final common pathway.
Differences in the muscle performance, output of the spinal motor neurons to the muscle, and the synaptic inputs to the motor neurons between persons with MS and healthy individuals are described in the next few paragraphs.
Muscle performance during strong contractions
An important outcome measure of the motor system is the maximal force that can be evoked in a muscle. The maximal evocable force can be determined with tetanic electrical nerve stimulation (5). Measurements of the maximal tetanic force in persons with MS have produced inconsistent results; tetanic force for persons with MS can be similar (25) or less than (8) that measured in control subjects. Even during maximal voluntary contractions (MVC), the results are inconsistent; MVC in persons with MS can be similar (29,32) or less than (28,30,31) that for control subjects. Some of this inconsistency can be attributed to variability within subject groups. When sex differences are considered, for example, a nonsignificant difference in MVC between MS and control groups can become significant (31). Moreover, as a consequence of deconditioning, strength is less preserved in muscles of the lower body than those of the upper body (26).
During sustained or repeated contractions, muscle force declines through time. The rate of decline in force during electrical nerve stimulation and voluntary activation can be used as an index of performance fatigability. Decreases in force during electrically evoked contractions are due to changes in the neuromuscular propagation and muscle fiber properties, whereas the reductions observed during voluntary contractions are caused by changes in one or more of the processes shown in Figure 1. A number of studies have found that the force decline during contractions evoked by electrical nerve stimulation is greater for persons with MS compared with control subjects, indicating that MS-related changes are present within the muscle fibers (8,28). The likely explanation for this difference is that individuals with MS typically are more sedentary, which means reduced levels of muscle activation and greater deconditioning of the muscles.
Studies that have examined performance fatigability during voluntary contractions have found increased (14,29,35) or similar levels of force decline (31,34) in persons with MS as compared with control subjects. When twitches were evoked by electrical nerve stimulation after the voluntary fatiguing contraction, most studies showed less of a decline in twitch force for persons with MS (29,31,32,35). The decrease in force during a sustained voluntary contraction, therefore, included a smaller contribution from force reduction of muscle fibers for persons with MS. Nevertheless, mechanisms underlying these differences in performance fatigability between persons with MS and control subjects can only be identified if additional measures of voluntary activation are obtained.
Voluntary activation of the muscle
Voluntary activation received by the muscle fibers (muscle activation, Fig. 1, step 2) is commonly measured with the superimposed twitch technique. A subject is asked to perform a maximal contraction and, during the contraction, the appropriate nerve is stimulated with pairs of electrical stimuli. If muscles are activated maximally by the nervous system, no additional force will be evoked by this stimulation. If not all muscle fibers are activated maximally by the nervous system, however, the stimulation will evoke extra force. This force increment is indicative of the difference between the maximal evocable force and the maximal voluntary force, and represents the difference in the actual activation level and maximal activation of the muscle by the CNS. Persons with MS exhibit equal (29,31) or reduced levels of voluntary activation compared with control subjects (8,21,30,35) during brief contractions. The reduced voluntary activation also is confirmed by low motor unit firing rates (21) in the quadriceps muscle of persons with MS. Voluntary activation during brief contractions has been found to be associated positively with the MVC force in persons with MS (31,35) demonstrating the importance of the voluntary activation for maximal force production in these individuals. The observed differences in voluntary activation likely depend on the phenotype of MS and the used muscles; persons with RRMS exhibiting greater activation than those with SPMS (35) and hand muscles and tibialis anterior having greater activation levels than the quadriceps. Interestingly, both hand muscles and tibialis anterior receive relatively strong corticospinal projections compared with the quadriceps.
The decline in voluntary activation during sustained contractions usually is greater for persons with MS than for control subjects (24,29–31,35). This greater decline in voluntary activation for persons with MS can be accompanied by either a greater (24,29,30,35) or similar (31,34,34) decrease in force compared with control subjects. A direct comparison between persons with RRMS and SPMS showed a greater force decline in those individuals with SPMS even after statistically correcting for the contribution of increased disability (35). In persons with MS, the decline in force and voluntary activation during sustained contractions were significantly associated in some studies (24,29,30,32) but not in others (18). These studies show that persons with MS, compared with control subjects, have greater difficulty in maintaining high levels of voluntary activation, and that the reduction in activation results in poorer muscle activation and therefore in a smaller decline in intrinsic muscle force. Whether the decrease in force during a sustained voluntary contraction is greater in persons with MS than in control persons depends on the relative reductions in voluntary activation and intrinsic force of the muscle fibers.
In contrast to persons with RRMS, those with SPMS demonstrate reduced voluntary activation even during brief maximal contractions (35). We interpret this observation as a sign of reduced capacity to compensate for disease-induced damage from the start of the contraction.
Use-dependent changes on spinal levels
Some of the decline in voluntary activation during a sustained contraction is attributable to adjustments that occur at the spinal level. The adjustments modulate the excitability of the motor neurons and mainly are due to changes in the intrinsic properties of motor neurons (10,16), but also inhibitory synaptic inputs provided by nociceptive and chemoceptive afferents (group III and IV) onto motor neurons (5). In addition to these inhibitory reflexes, there is a progressive decline in excitatory feedback from muscle spindles that makes it even more difficult to maintain high firing rates (5).
It is not expected that either motor neuron properties or peripheral afferent projections would be changed substantially in persons with MS, although the observation that some individuals exhibit an increased incidence of muscle spasms suggest some changes in motor neuron excitability. Nevertheless, the reduction in motor neuron excitability during sustained voluntary contractions requires an increase in the voluntary drive to the motor neuron pool to maintain the same force output. This means that persons with RRMS, who are able to compensate for disease-induced damage during the brief contractions, might not be able to sustain the target force when additional voluntary drive is required to compensate for the adjustments at spinal levels. Persons with SPMS were not able to compensate for the disease-induced changes from the start of the contraction resulting in a greater force decline than persons with RRMS.
Corticospinal excitability
The activation signals sent from the primary motor cortex to the muscle fibers can be assessed with transcranial magnetic stimulation (TMS) by eliciting motor evoked potentials (MEP) in limb muscles. The central conduction velocity of action potentials can be calculated, serving as a measure of the integrity of the descending pathways; primarily, the integrity of the fast conducting fibers in the corticospinal tract.
In general, MEP conduction velocity is slower in persons with MS compared with control subjects (31). The reduced MEP conduction velocity increases the variability in the arrival times of the corticospinal input onto the motor neurons, thereby decreasing the efficacy of this input. Approximately half of the studies have found a reduction in the excitability of the corticospinal tract as indicated by a small increase in resting motor threshold for TMS responses and a reduced MEP amplitude (38); however, some studies did not find any difference in resting motor threshold (14,31,34) or MEP amplitude (14,31). These differences were at least partly related to MS phenotype; most studies showed that TMS measures were more affected in persons with SPMS than those with RRMS (38). There do not seem to be any consistent associations between TMS measures at rest and the level of fatigue reported by individuals with MS.
Supraspinal activity
Functional magnetic resonance imaging (fMRI) can be used to visualize changes in the ratio between oxygenated and deoxygenated hemoglobin with blood oxygenation level dependent (BOLD) contrast and provides an index of neuronal activity. When performing unimanual tasks, the activation signal mainly arises from motor areas in the hemisphere contralateral to the moving hand. Activation increases in primary and secondary motor areas (supplementary motor area, premotor cortex, cingulate areas) during both complex motor tasks and effortful contractions, and this activation tends to spread into connected areas including primary and secondary motor areas in the ipsilateral hemisphere. In persons with MS, there is both fMRI and TMS evidence demonstrating that this increased ipsilateral activity is already present during the performance of submaximal or simple motor tasks (13,14,22,34).
Furthermore, some experiments have examined areas that were associated with self-reported fatigue and found differences in activation during (nonfatiguing) motor tasks (see for recent reviews (2,9,19)). Areas that showed increased activation included the ipsilateral cerebellum, rolandic operculum, thalamus, anterior cingulate cortex, basal ganglia, parietal cortex, and orbitofrontal cortex. Consistent with these changes, TMS measures have revealed decreased short-interval intracortical inhibition in persons with MS (14,38) although without changes in intracortical facilitation (14). Findings on the levels of interhemispheric inhibition, however, are less consistent (38). Overall, these experiments suggest that persons with MS are able to provide the same amount of output to the motor neuron pool during submaximal motor tasks compared with control subjects, but this requires increased levels of ipsilateral cortical activation. Nonetheless, the activation in primary motor areas was not related to self-reported levels of fatigue in persons with MS.
In control subjects, sustained maximal contractions involve a progressive increase in the activation of cortical motor areas including ipsilateral motor areas (20). In contrast with brief maximal contractions, individuals with RRMS exhibited an attenuated increase in both contralateral and ipsilateral activation during sustained contractions; the intensity of the BOLD signal increased in the first 60 s of the maximal contraction performed by persons with RRMS, but declined in the second 60 s of the fatiguing contraction (32). The total amount of activation during the 120-s contraction was reduced significantly in the ipsilateral sensory-motor cortex of persons with RRMS.
The increase in cortical motor activation in control subjects likely reflects a compensatory increase in the voluntary drive to the motor neuron pool to maintain maximal force output. If the ipsilateral activation also reflects compensatory adjustments, the aforementioned observation suggests that during submaximal contractions persons with RRMS already use this compensatory activation. Whereas control subjects can engage this compensatory activation during fatiguing contractions, this is not possible for persons with MS and hastens the decrease in muscle activation.
Alternatively, activation of ipsilateral areas could reflect degenerative alterations rather than compensatory activation. The corpus callosum is the largest white matter structure in the brain and this structure is especially vulnerable in persons with RRMS (6). The corpus callosum largely relays inhibitory connections between the two hemispheres, and the loss of integrity among its fibers augments ipsilateral activation during voluntary contractions. Consequently, individuals with more ipsilateral activation will have greater difficulty with tasks that require strong interhemispheric communication. Similar observations of increased ipsilateral activation have been reported for elderly adults. If ipsilateral activation represents a compensatory strategy, this option is reduced with advancing age for persons with MS.
The previous paragraphs describe the use- and diseaseinduced functional changes in the CNS and muscles of persons with MS during brief and sustained contractions. As with disease progression, the functional consequences can be quite variable. When sustaining a maximal contraction, for example, the decline in force exhibited by persons with MS can be greater than or similar to that observed for control subjects. Our findings indicate that the eventual force decline (performance fatigability) experienced by individuals with MS mainly depends on the ability to maintain a maximal voluntary drive by compensating for use- and disease-related changes.
Associations Between Fatigue and Fatigability
In the preceding paragraphs, we have argued that performance fatigability in persons with MS could be the result of a reduced capacity to compensate for use- and disease-related changes. Measures of perceived fatigability in persons with MS likely are most affected by (over)activity of the immune system (1,4,17). The pro- and anti-inflammatory cytokines released by activated microglia cells reduce the homeostatic and repair function of these immune cells in the CNS. Behavioral consequences of (over)activation of these cells often are described as sickness behavior and include increased levels of fatigue. Furthermore, the metabolism and availability of the neurotransmitters serotonin, dopamine, norepinephrine, and glutamate are affected seriously by proinflammatory cytokines resulting in additional changes in motivation, perception of effort, and depression (1,4,17).
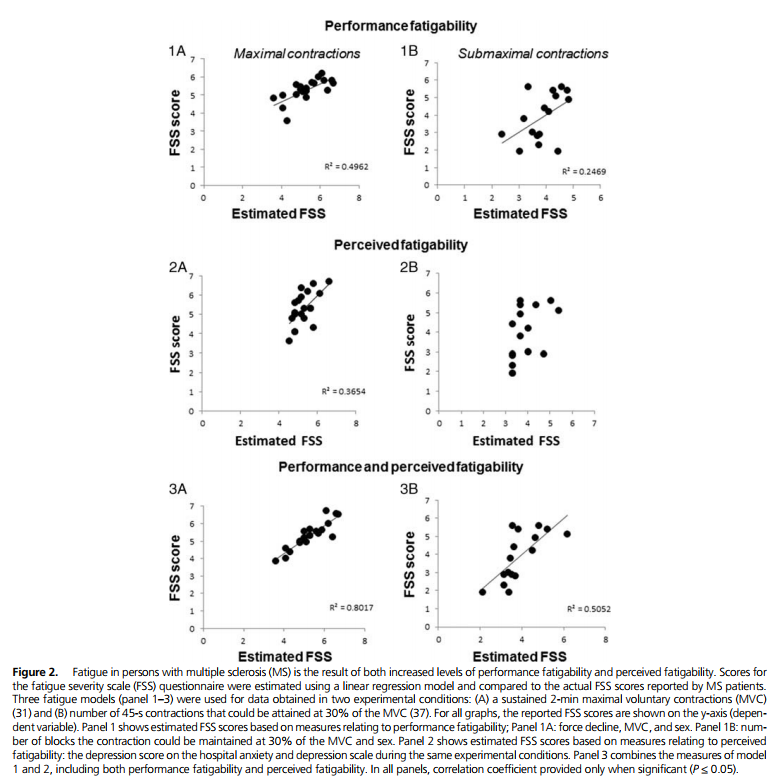
Yet, the fatigue reported by individuals with MS is a subjective feeling and can only be quantified by self-reported questionnaires. So, how does performance fatigability and perceived fatigability in persons with MS relate to fatigue? Several experiments have examined the association between fatigue and performance fatigability during fatiguing motor tasks, and, frequently, no or weak associations have been found (36). However, our experiments have revealed three examples for persons with RRMS in which there was a significant association between fatigue (measured with the fatigue severity scale (FSS)) and force decline (31,36) or time to task failure (37); however, no such association has been found for persons with SPMS (35). Significantly, the association between fatigue and fatigability was quantified with a model that included both measures of depression (a variable that reflects perceived fatigability) and force decline (performance fatigability). This model (Fig. 2)was able to explain a major part of the variance in the fatigue scores measured with the FSS.
One question that remains is whether the increased levels of fatigue and fatigability for persons with MS are related to structural damage to specific areas in the brain. One approach to this question has been to examine the association between structural damage — white and grey matter lesion load or atrophy — and the level of fatigue. The results are largely ambiguous (see recent reviews by (2,9,19)), but one study that enrolled a large number of subjects did find associations between total lesion load or atrophy and fatigue scores (33). When significant associations have been reported, the structural changes were observed in the frontal lobe, corpus callosum (2), prefrontal cortex (23), and right parieto-temporal lobe (2,9,19,27). However, these structural data should be interpreted with caution because fatigue often is associated with increased depression scores (measure of perceived fatigability). Indeed, a large study found that indices of depression and fatigue in persons with MS involved overlapping areas in the brain (7). Nonetheless, most of the areas in which structural damage is associated with fatigue are involved in processes related to attention, homeostasis, or assessing the sense of effort. These associations will be difficult to disentangle, however, because persons with MS report a more rapid increase in the ratings of perceived exertions during a fatiguing task (34). Yet, there is no significant association between perception of effort and fatigue in persons with MS (34). Nevertheless, MS-induced structural and functional changes in cortical and subcortical areas involved in homeostasis and interoception are likely to elevate levels of perceived fatigability and consequently increase MS-related fatigue.
CONCLUSIONS
MS is considered an inflammatory autoimmune disorder of the CNS resulting in axonal demyelination. Axonal demyelination and consequently neuronal dysfunction are signs of deterioration induced by the (over)activation of the immune system resulting in compensatory activation within networks of the CNS. All these structural and functional changes have sensory, motor, and cognitive consequences including increased levels of fatigue. Fatigue in individuals with MS is the consequence of both increased levels of performance fatigability and perceived fatigability.
Increased levels of performance fatigability are illustrated by the difficulty that persons with MS have with maintenance of maximal voluntary activation. In persons with RRMS, measures of performance fatigability during sustained maximal contractions (decline in normalized force and voluntary activation) were associated with fatigue as indexed with a questionnaire. Because reduced voluntary activation coincides with the attenuation of the compensatory activation in cortical brain areas, fatigue could reflect the failure of adequate cortical and subcortical brain activation.
Fatigue in persons with MS also relates to structural damage in areas that likely are involved in attentional processes, homeostasis, or networks underlying the sense of effort; that is, processes that are captured by measures of perceived fatigability. Variation in fatigue among RRMS patients could indeed be explained better when adding a measure of perceived fatigability (depression scores indexed with a questionnaire) in the explanatory model demonstrating the importance of including both dimensions to explain differences in fatigue in persons with MS.
REFERENCES
1. Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011; 130:226–38.
2. Chalah MA, Riachi N, Ahdab R, Creange A, Lefaucheur JP, Ayache SS. Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front. Cell. Neurosci. 2015; 9:460.
3. Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372:1502–17.
4. Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014; 37:39–46.
5. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001; 81:1725–89.
6. Giorgio A, De Stefano N. Advanced structural and functional brain MRI in multiple sclerosis. Semin. Neurol. 2016; 36:163–76.
7. Gobbi C, Rocca MA, Riccitelli G, et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult. Scler. 2014; 20:192–201. 8. de Haan A, de Ruiter CJ, van de Woude LH, Jongen PJ. Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve. 2000; 23:1534–41.
9. Hanken K, Eling P, Hildebrandt H. Is there a cognitive signature for MS-related fatigue? Mult. Scler. 2015; 21:376–81.
10. Kernell D. The Motoneurone and its Muscle Fibres. Oxford (England): Oxford University Press; 2006.
11. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013; 80:409–16.
12. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch. Neurol. 1988; 45:435–7.
13. Lenzi D, Conte A, Mainero C, et al. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum. Brain Mapp. 2007; 28:636–44. 14. Liepert J, Mingers D, Heesen C, Baumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult. Scler. 2005; 11:316–21.
15. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014; 83:278–86.
16. McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC, Taylor JL. The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J. Physiol. 2011; 589(Pt 15):3731–8.
17. Morris G, Berk M, Galecki P, Walder K, Maes M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol. Neurobiol. 2015; 53:1195–219.
18. Ng AV, Miller RG, Kent-Braun JA. Central motor drive is increased during voluntary muscle contractions in multiple sclerosis. Muscle Nerve. 1997; 20:1213–8.
19. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun. Rev. 2016; 15:210–20.
20. Post M, Steens A, Renken R, Maurits NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: an fMRI study. Hum. Brain Mapp. 2009; 30:1014–27.
21. Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve. 1992; 15:1123–32.
22. Rocca MA, Colombo B, Falini A, et al. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005; 4:618–26.
23. Roelcke U, Kappos L, Lechner-Scott J, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997; 48:1566–71.
24. Romani A, Bergamaschi R, Candeloro E, Alfonsi E, Callieco R, Cosi V. Fatigue in multiple sclerosis: multidimensional assessment and response to symptomatic treatment. Mult. Scler. 2004; 10:462–8.
25. de Ruiter CJ, Jongen PJ, van de Woude LH, de Haan A. Contractile speed and fatigue of adductor pollicis muscle in multiple sclerosis. Muscle Nerve. 2001; 24:1173–80.
26. Schwid SR, Thornton CA, Pandya S, et al. Quantitative assessment of motor fatigue and strength in MS. Neurology. 1999; 53:743–50.
27. Sepulcre J, Masdeu JC, Goni J, et al. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult. Scler. 2009; 15:337–44.
28. Sharma KR, Kent-Braun J, Mynhier MA, Weiner MW, Miller RG. Evidence of an abnormal intramuscular component of fatigue in multiple sclerosis. Muscle Nerve. 1995; 18:1403–11.
29. Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain. 1997; 120(pt 2):299–315.
30. Skurvydas A, Brazaitis M, Andrejeva J, Mickeviciene D, Streckis V. The effect of multiple sclerosis and gender on central and peripheral fatigue during 2-min MVC. Clin. Neurophysiol. 2011; 122:767–76.
31. Steens A, de Vries A, Hemmen J, et al. Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil. Neural Repair. 2012; 26:48–57.
32. Steens A, Heersema DJ, Maurits NM, Renken RJ, Zijdewind I. Mechanisms underlying muscle fatigue differ between multiple sclerosis patients and controls: a combined electrophysiological and neuroimaging study. Neuroimage. 2012; 59:3110–8.
33. Tedeschi G, Dinacci D, Lavorgna L, et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J. Neurol. Sci. 2007; 263:15–9.
34. Thickbroom GW, Sacco P, Kermode AG, et al. Central motor drive and perception of effort during fatigue in multiple sclerosis. J. Neurol. 2006; 253:1048–53.
35. Wolkorte R, Heersema DJ, Zijdewind I. Reduced voluntary activation during brief and sustained contractions of a hand muscle in secondaryprogressive multiple sclerosis patients. Neurorehabil. Neural Repair. 2016; 30:307–16.
36. Wolkorte R, Heersema DJ, Zijdewind I. Muscle fatigability during a sustained index finger abduction and depression scores are associated with perceived fatigue in patients with relapsing-remitting multiple sclerosis. Neurorehabil. Neural Repair. 2015; 29:796–802.
37. Wolkorte R, Heersema DJ, Zijdewind I. Reduced dual-task performance in MS patients is further decreased by muscle fatigue. Neurorehabil. Neural Repair. 2015; 29:424–35.
38. Yusuf A, Koski L. A qualitative review of the neurophysiological underpinnings of fatigue in multiple sclerosis. J. Neurol. Sci. 2013; 330:4–9.


