Diet quality
Why is this study important?
Most of us, even the most dedicated coach potatoes, will generally agree that a healthy diet, maintaining a healthy weight, getting regular exercise, and cutting back on alcohol and smoking makes for a healthier lifestyle. But what about people with MS? Do these factors, the authors wondered, also help individuals with MS to reduce their level of disability as well as the intensity of their symptoms?
To help to answer this, the authors surveyed an impressive 6,989 individuals who had received a diagnosis of MS from a physician. The study population was not particularly diverse: 92% were Caucasian; most were married (42%), older (mean age of 59.5 years); and had lived with MS for just under 10 years. That said, the conclusions from a study population this large have a great deal of credibility.
What defines "a healthy diet?"
The authors established four criteria:
- High consumption of fruits, vegetables, and legumes
- High consumption of whole grains
- Limited consumption of foods with added sugars (such as sugar-sweetened beverages and dessert foods)
- Limited consumption of red and processed meats
The authors developed an overall diet quality score for each individual based on these food groups. They then studied the association between diet quality and disability status.
...But is it sufficient to assess diet alone?
No, it is not: a healthy diet is beneficial, but how much it benefits each individual depends on such additional factors as age, weight, exercise habits, and smoking habits. The investigators therefore decided to study as well the impact of healthy diet as part of an overall healthy lifestyle. Using American Heart Association guidelines, the authors defined a healthy lifestyle as one where an individual maintains a healthy weight (BMI <25), routinely engages in physical activity, abstains from smoking, and consumes a better-than-average diet.
Finally, the authors wondered whether people with MS who maintained a healthy lifestyle experienced a milder symptoms of disability, depression, fatigue, pain, or cognitive impairment than those who did not practice a healthy lifestyle.
What did their study show?
The results suggest that a healthy diet does not appear to reduce the severity of disability, fatigue, pain, or cognitive impairment. Individuals who stick to a healthy diet, however, tend to struggle less frequently with depression. Also, when the investigators looked at an overall healthy lifestyle (including healthy diet), they found that individuals who practice a healthy lifestyle appear to be at lower risk for severe depression, pain, fatigue, and cognitive problems. Moreover, the results suggest that people with MS who maintain an overall healthy lifestyle tend to experience milder levels of disability. The investigators noted that whole grains and dairy products, in particular, seemed to be associated with milder disability.
What's the bottom line?
High-quality diets emphasizing fruits, vegetables and legumes, and whole grains, and low consumption of sugar and red meat, the study results suggest, are associated with lower levels of disability in people with MS. Furthermore, individuals who maintain a healthier lifestyle appear to have a lower prevalence of severe depression, pain, fatigue, and cognitive problems. Because diet and other lifestyle elements are something people with MS can control, they may offer another way to modify the course of their disease.
As with all changes such as this, talk to your health care provider about your interest in making healthy choices in your diet and beginning an exercise program.
Original Article
Diet quality is associated with disability and symptom severity in multiple sclerosis
Kathryn C. Fitzgerald, ScD, Tuula Tyry, PhD, Amber Salter, PhD, Stacey S. Cofield, PhD, Gary Cutter, PhD, Robert Fox, MD, and Ruth Ann Marrie, MD, PhD
Neurology®
Abstract
Objective To assess the association between diet quality and intake of specific foods with disability and symptom severity in people with multiple sclerosis (MS).
Methods In 2015, participants in the North American Research Committee on MS (NARCOMS) Registry completed a dietary screener questionnaire that estimates intake of fruits, vegetables and legumes, whole grains, added sugars, and red/processed meats. We constructed an overall diet quality score for each individual based on these food groups; higher scores denoted a healthier diet. We assessed the association between diet quality and disability status as measured using Patient-Determined Disease Steps (PDDS) and symptom severity using proportional odds models, adjusting for age, sex, income, body mass index, smoking status, and disease duration. We assessed whether a composite healthy lifestyle measure, a healthier diet, healthy weight (body mass index <25), routine physical activity, and abstinence from smoking was associated with symptom severity.
Results Of the 7,639 (68%) responders, 6,989 reported physician-diagnosed MS and provided dietary information. Participants with diet quality scores in the highest quintile had lower levels of disability (PDDS; proportional odds ratio [OR] for Q5 vs Q1 0.80; 95% confidence interval [CI] 0.69–0.93) and lower depression scores (proportional OR for Q5 vs Q1 0.82; 95% CI 0.70–0.97). Individuals reporting a composite healthy lifestyle had lower odds of reporting severe fatigue (0.69; 95% CI 0.59–0.81), depression (0.53; 95% CI 0.43–0.66), pain (0.56; 95% CI 0.48–0.67), or cognitive impairment (0.67; 95% CI 0.55–0.79).
Conclusions Our large cross-sectional survey suggests a healthy diet and a composite healthy lifestyle are associated with lesser disability and symptom burden in MS.
Multiple sclerosis (MS) is a disease of the CNS1; it remains unclear why certain individuals experience a mild course with little disability progression while others accumulate substantial disability.1,2 Diet is a potentially modifiable contributor to disease progression.3 Several MS-specific diets have been popularized as reducing symptoms and ameliorating disability, including the low saturated fat Swank diet and the modified Paleolithic Wahls diet.4,5 While anecdotes support specific dietary modifications improving MS symptoms, few studies have thoroughly evaluated these hypotheses. Prior studies were small, did not include detailed information regarding symptom severity, or failed to address the association between diet and other lifestyle characteristics.
Therefore, we evaluated the association between diet, including overall diet quality and individual components, and disability and symptom severity, while accounting for other lifestyle factors. Since healthy diets are associated with lower levels of pain, fatigue, cognitive impairment, and depressive symptoms in healthy individuals,6,7 we also evaluated whether similar associations exist in people with MS. Finally, we considered how diet and other modifiable lifestyle factors such as smoking, physical activity, and obesity may collectively be associated with symptom severity.
Methods
Study population
We included participants in the North American Research Committee on MS (NARCOMS) Registry. The registry includes >38,000 individuals with self-reported MS registered since 1996; 11,011 are active participants (having responded to a questionnaire in the last 2 years). A prior validation study confirmed diagnoses of MS in >98% of sampled participants.8 At enrollment, participants report date of birth, sex, and age at MS symptom onset. On semi-annual update surveys, participants report smoking status, alcohol intake, participation in leisure time physical activity, annual household income, and clinical and disease characteristics, including height and weight (used to calculate body mass index [BMI]), diseasemodifying therapy use in the last 6 months (yes, no), MS-related disease status, symptoms, and disability.
Standard protocol approvals, registrations, and patient consents
Participants consent to use of their de-identified information for research. The NARCOMS registry is approved by the Institutional Review Board at the University of Alabama at Birmingham.
Disease activity, progression, and symptom severity
Participants reported whether they had experienced a relapse in the previous 6 months and whether they had experienced a gradual worsening of symptoms in those 6 months. They reported disability using Patient-Determined Disease Steps (PDDS), which strongly correlates with clinician-assessed measures, such as the Expanded Disability Status Scale (r = 0.78).9 Using Performance Scales,10 participants reported impairment in 8 domains, including mobility, hand function, vision, fatigue, cognition, bladder/bowel, sensory, and spasticity. Participants also reported impairment in additional domains including depression, tremor, and pain. Except for mobility (which is a 7-point scale), each domain is rated from 0 (normal) to 5 (total disability). The validity of these scales has been demonstrated previously.10–16
Dietary information
Participants completed a dietary screener questionnaire (DSQ), developed by the National Health and Nutrition Examination Survey (NHANES) in 2009–2010.17 The DSQ provides an overview of diet when comprehensive information from extensive surveys or 24-hour recalls is not feasible; each of the DSQ’s 26 questions was selected for its relationship to one or more dietary factors. The DSQ captures intakes of fruits, vegetables and legumes, dairy/calcium, added sugars (including those from sugar-sweetened beverages and dessert foods), whole grains/fiber, and red/processed meat by applying age- and sex-adjusted scoring algorithms to convert screener responses to estimates of total dietary intake for these dietary items. The measures used in the scoring algorithm were estimated from the more comprehensive evaluation of diet from an analysis of the full dietary information completed by NHANES participants.17
We developed a diet quality score as a singular, comprehensive measure of a person’s overall diet quality using the predicted intakes of DSQ food group categories: (1) fruits, vegetables, and legumes, (2) whole grains, (3) sugar from desserts and sweetened beverages, and (4) red and processed meats. These food groups were selected based on studies of other chronic diseases.18–20 To develop the score, we used a quintile-scoring approach applied in previous studies of heart disease and other chronic diseases.21–24 We assigned individuals to sex-specific approximate quintiles based on their intake of a specific food group. Component scores for fruits, vegetables and legumes, nuts, and whole grains were assigned using the individual’s quintile ranking for that item. For red and processedmeats and added sugar intake, lower intakes are desired, so individuals in the lowest quintile received a score of 5, whereas individuals in the highest quintile received a score of 1. We did not include intake of dairy foods because the DSQ does not distinguish different types of dairy, which may have differing health effects (e.g., high vs no saturated fat dairy). The composite score was the sum of the component scores, and ranged from 4 (low overall diet quality) to 20 (high overall diet quality). Questions corresponding to each subpart of the diet score are available in table e-1, http://links.lww.com/WNL/A13.
Participants reported whether they currently or previously followed any of 19 specific diets since being diagnosed with MS, including MS-specific diets (Swank, Wahls), popular diets (Paleo, Atkins, The Zone Diet, South Beach, Dukan, raw food, juice cleanses, or weight-loss plans like Jenny Craig, Weight Watchers, Nutrisystem), and more general diets (DASH-style diet, Mediterranean, vegetarian, pescatarian, vegan, gluten-free, low-calorie, low-carbohydrate, or lowsugar). They also reported the reason for following a specific diet as general health, weight loss, or MS.
Composite healthy lifestyle
As poor dietary quality tends to correlate with other unhealthy lifestyle characteristics, we assessed whether a similar association existed in people with MS using indicators of a composite healthy lifestyle adapted from guidelines set by the American Heart Association.25,26 We defined a composite healthy lifestyle as one where an individual maintains a healthy weight (BMI <25), routinely engages in physical activity, abstains from smoking, and consumes a better than average diet (> median diet quality score). Routine physical activity was defined as participating in physical activity or exercise (running, calisthenics, golf, gardening, or walking for exercise) in the last month.
Statistical analysis
We excluded responders who did not report physician confirmed MS (n = 47), were unsure of theirMS diagnosis type or reported having other diseases (n = 174), or did not provide any information on diet or age (n = 378). We summarized sample characteristics using mean (SD), median (interquartile range [IQR]), and frequency (%) as appropriate.
We assessed the association between the PDDS, depression, pain, fatigue, and cognition and our diet quality score and individual diet components using proportional odds models. We assessed deviations from proportionality (nonparallel lines) for included covariates graphically and using likelihood ratio tests. When this assumption indicated a potential violation, we conducted sensitivity analyses allowing for nonproportionality (for violating covariates) using partial-proportional odds models. We fit similar multinomial regression models assessing the relation between overall diet quality scores and individual dietary component (DSQ foods) and disability severity by categorizing PDDS as mild (PDDS <2), moderate (PDDS 2–5), or severe (PDDS ≥6). Analyses of diet quality and depression, pain, fatigue, and cognition were adjusted for disability (PDDS categories).We adjusted for covariates in all models: age (continuous), disease duration (quartiles: <13 [ref], 13–18, 18–25, ≥25 years), BMI (quartiles: <22.5 [ref], 22.5–25.8, 25.9–30.3, ≥30.4), income (<$50,000 [ref], $50–100,000, ≥$100,000, decline to answer), and smoking status (no [ref], yes). We adjusted for missing covariate information using indicator variables.
We assessed the association between prevalence of relapse in the previous 6 months (binary; yes, no) and gradual feeling of symptoms worsening (binary; yes, no) with our diet quality score using logistic regression. Since the prevalence of relapse decreases with increasing age, we fit additional models stratified by age quartile. Additional analyses stratified by MS course (relapsing-remitting, secondary progressive, and primary progressive), smoking status, and whether individuals were following a specific diet for MS (yes, no).
Finally, we assessed whether composite healthy lifestyle was associated with disability, depression, fatigue, pain, or cognitive impairment.We applied multinomial models for mild, moderate, and severe impairment defined using the following score categorizations: disability (mild, moderate, severe, as defined above), depression/cognition/fatigue (mild 0, moderate 1–2, severe ≥3), and pain (mild 0–1, moderate 2–3, severe ≥4). Models were adjusted for age, sex, income, and disease duration. Models for depression, pain, fatigue, and cognition also adjusted PDDS score. Because disability may limit physical activity, sensitivity analyses created a composite healthy lifestyle considering only smoking status, weight, and diet.
Analyses were conducted using SAS version 9.4 (Cary, NC) and R version 3.2.2 (r-project.org/).
Results
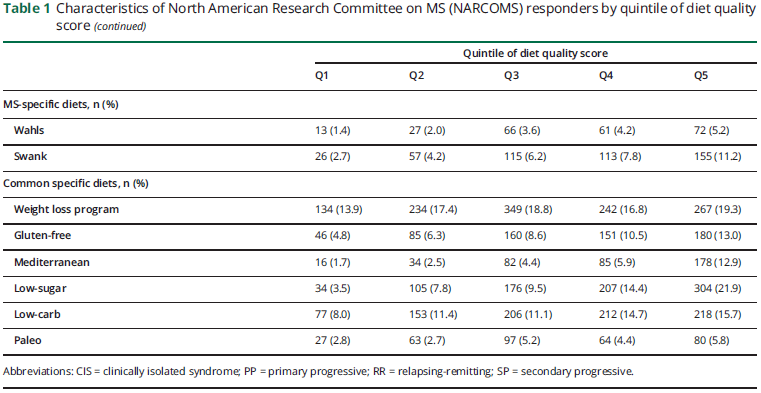
Of the 11,100 active registry participants, 7,639 (69%) individuals responded to the fall 2015 survey and 6,989 were included in the analysis; their characteristics are shown in table 1. As compared to nonresponders, responders weremore likely to be Caucasian (92% vs 88%), married (41.9% vs 31.0%), older (mean [SD] 59.5 [13.1] years vs 56.0 [33.4] years), have longer disease duration (19.7 [9.9] years vs 16.5 [10.5] years), and be longer-time study participants (11 [5.4] years vs 8 [6.3] years). Responders and nonresponders had similar disability at enrollment (median PDDS [IQR] 3 [1–4] vs 3 [1–4]).
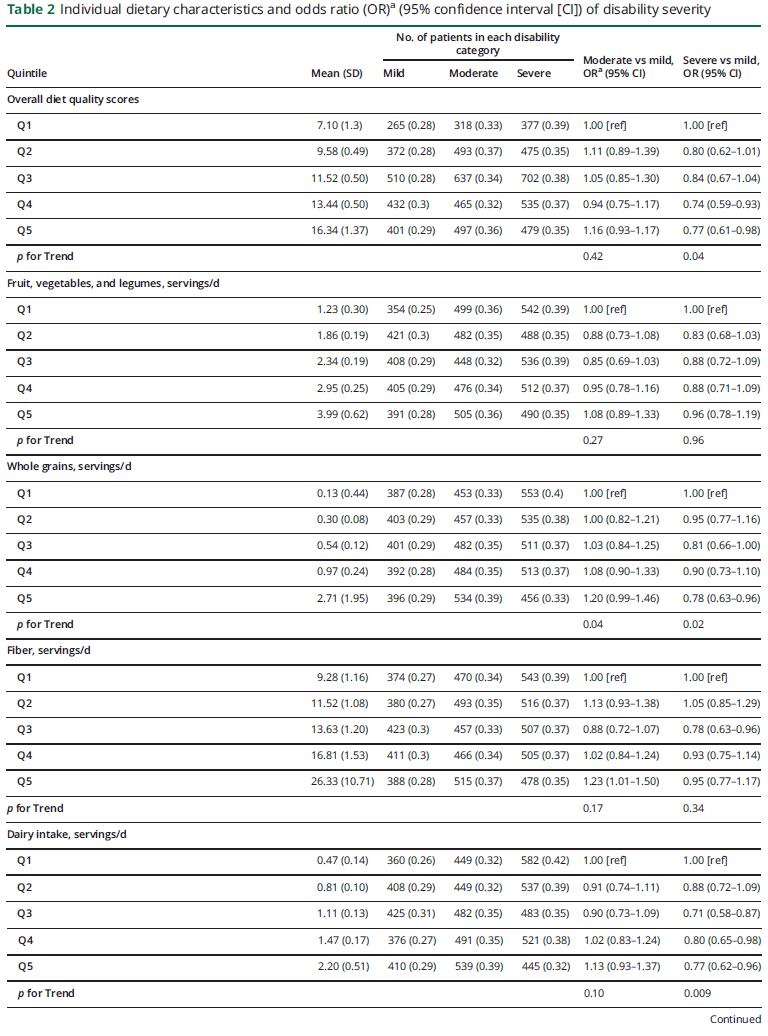
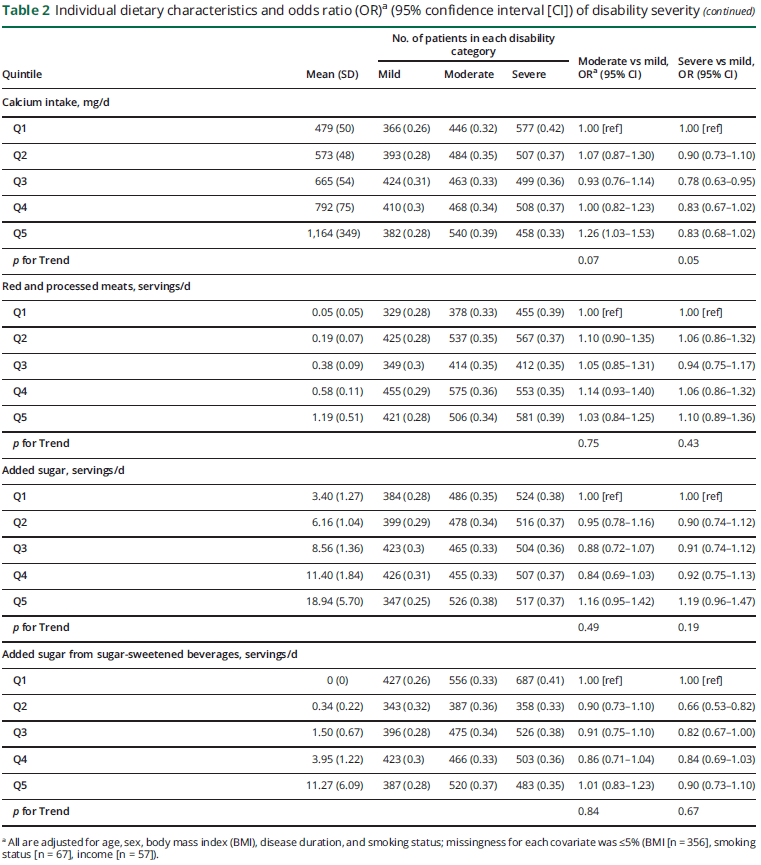
Overall diet quality scores averaged 11.9 (SD 3.0) and ranged from 4 to 20. Individuals with higher diet quality scores were older, were slightly more likely to be Caucasian, and had higher levels of income (table 2). Those with higher diet quality scores were less likely to be smokers and be less overweight and were more likely to participate in physical activity and report following a special diet forMS. Diet quality scores did not vary appreciably across clinical MS characteristics.
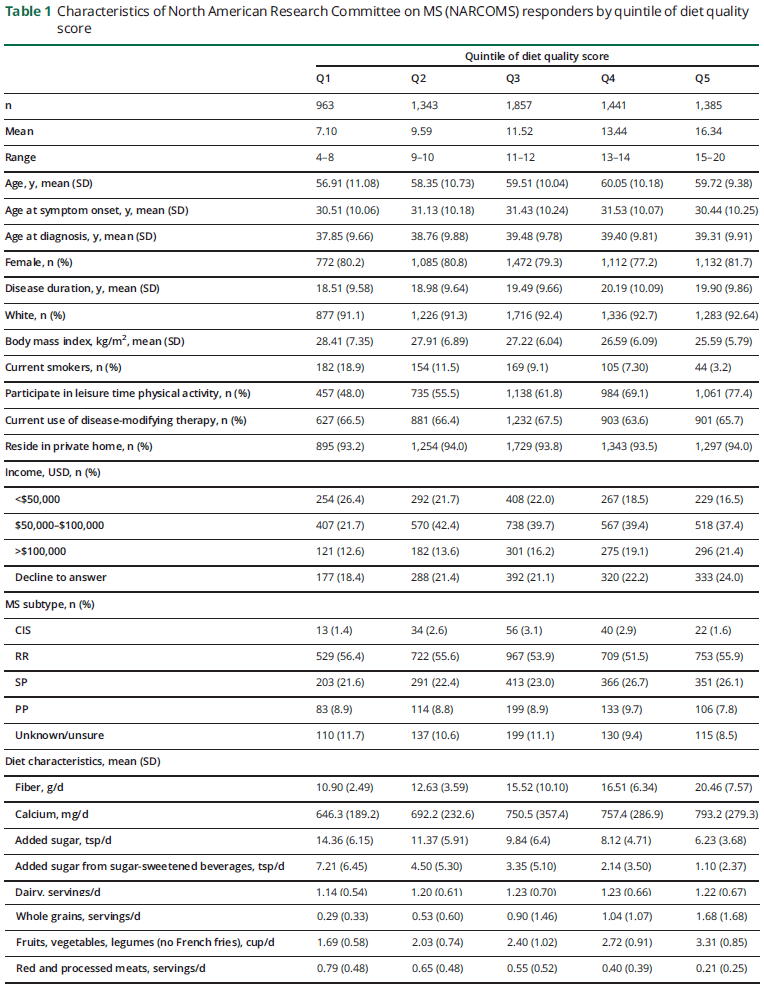
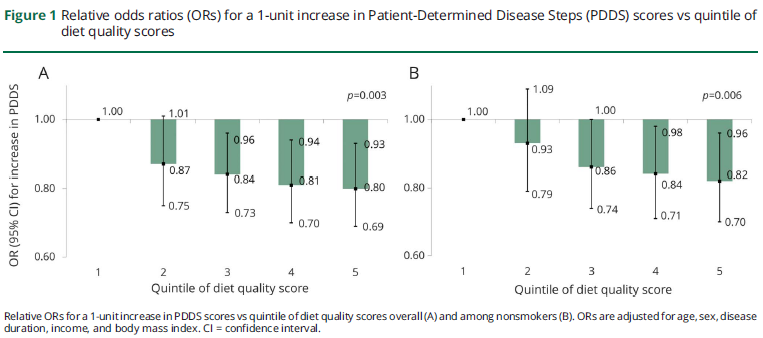
In multivariable proportional odds models adjusted for age, sex, household income, disease duration, BMI, and smoking status, individuals in the top quintile of diet quality score were at 20% lower odds of higher PDDS scores relative to individuals in the bottom quintile (figure 1A; odds ratio [OR] 0.80; 95% confidence interval [CI] 0.69–0.93; p for trend = 0.002). We observed similar results using multinomial models where individuals in the top quintile had a 21% lower prevalence of severe vs mild disability than those in the bottom quintiles (OR 0.77; 95% CI 0.61–0.98; p for trend = 0.04; table 2). Since smoking is associated with diet quality and disability status,27 we assessed the association between diet quality and disability status among nonsmokers (figure 1B). Findings were consistent; individuals in the top quintile were at 17% lower odds of higher PDDS scores when compared with those in the bottom quintile (OR 0.82; 95% CI 0.70 to 0.96; p for trend = 0.006). In the analyses of the specific diets queried, any exposure (past or current) to one of the diets or diet plans was associated with modestly lower odds of increasing disability (OR 0.89; 95% CI 0.81–0.97; p = 0.009). Individually, exposure to the Wahls diet or a gluten-free diet was associated with greater disability (Wahls OR 1.51; 95% CI 1.25–1.78; gluten-free OR 1.31; 95% CI 1.13–1.52). However, this association is likely driven by differences in the prevalence of progressive disease subtypes in individuals following this diet; individuals following theWahls diet were nearly 50% more likely to have progressive MS. Exposure to a weight loss plan diet was associated with lower disability (0.88; 95% CI 0.79–0.99), while exposure to other diets was not associated with disability.
In the individual food group analyses (table 2), individuals in the top quintile of intake of whole grains and of total dairy were at lower odds of severe vs mild disability than those in the bottom quintile of each food group (whole grains, Q5 vs Q1: OR 0.78; 95% CI 0.63–0.96; p for trend = 0.02; dairy intake, Q5 vs Q1: OR 0.77; 95% CI 0.62–0.96; p for trend = 0.009). We observed consistent results using proportional odds models (data not shown). Other dietary components were not associated with disability status.
Higher diet quality was associated with lower odds of more severe depressive symptoms in multivariable models adjusting for disability status (table 3; Q5 vs Q1: 0.82; 95% CI 0.70–0.97; p for trend = 0.01). Diet quality was not associated with severity of fatigue, pain, or cognitive symptoms. Similarly, for the remaining symptom domains, except for mobility (which correlates highly with PDDS), diet quality scores were not associated with symptom severity (table e-2, http://links.lww.com/WNL/A13).
As compared to individuals who did not, those who adhered to a composite healthy lifestyle were at substantially lower odds of severe vs mild depression (OR 0.53; 95% CI 0.43–0.66), pain (OR 0.56; 95% CI 0.48–0.67), fatigue (OR 0.69; 95% CI 0.59–0.81), and cognitive symptoms (OR 0.66; 95% CI 0.43–0.66), after adjusting for disease duration, PDDS, age, and sex (figure 2). A composite healthy lifestyle was also associated with lower odds of severe disability vsmild disability (OR 0.45; 95% CI 0.38 to 0.52; p < 0.0001). In sensitivity analyses that excluded physical activity from the composite healthy lifestyle, participants who maintained a composite healthy lifestyle remained at lower odds of severe disability vs mild disability (OR 0.83; 95% CI 0.72–0.95; p = 0.008).
Diet quality scores were not associated with relapse in the previous 6 months (Q5 vs Q1: 0.94; 95% CI 0.75–1.17) or with gradual symptom worsening (Q5 vs Q1: 0.91; 95% CI 0.76–1.08).
Discussion
In this large survey of diet quality and MS symptoms, diets higher overall in fruits, vegetables, legumes, and whole grains and lower in added sugars from sweets and sugar-sweetened beverages and red meat were associated with lower disability levels. Higher intakes of whole grains and dairy products were associated with lower disability. Higher overall diet quality was also associated with less severe depression. A composite healthy lifestyle was associated with less severe depression, pain, fatigue, cognitive impairment, and disability.
Our findings are consistent with smaller cross-sectional studies of diet in people with MS. Low fruit and vegetable intake was associated with lower quality of life in Southern Australians with MS.28 A global study found that a diet emphasizing higher intakes of fruits and vegetables, omega-3 fatty acids, and fiber, and lower intakes of sodium and alcohol, was also associated with higher physical andmental composite health.29 In those studies, as in this one, causal inference cannot be made because poor physical or mental health may lead to poorer diet quality or food choices. While the mechanisms linking diet to disability in people with MS are unknown, diet can influence gut microbiota, immune status, and burden of oxidative stress.30–32
In people with MS, few longitudinal studies of diet have been conducted, and several small randomized trials of dietary modifications are ongoing or have recently been completed. One trial testing early vs delayed randomization to a very-low-fat, plant-based diet in 61 people with MS did not find improvements in brain MRI outcomes or relapse rate over 1 year.33 However, the study was not powered to detect differences in these outcomes and observed an improvement in fatigue, cholesterol, and insulin levels. Three ongoing trials with sample sizes ranging from 30 to 45 participants are assessing feasibility of diets such as intermittent fasting or a healthy American-style (ClinicalTrials.gov identifier: NCT02647502, NCT02986893) and another study on the Wahls diet or the Wahls Paleo Plus vs the Swank diet (NCT02914964), where the primary outcome is fatigue. These trials are not designed to determine the long-term disease-modifying effects of these diets. Additional longitudinal observational and interventional studies that evaluate a range of outcomes will still be needed.
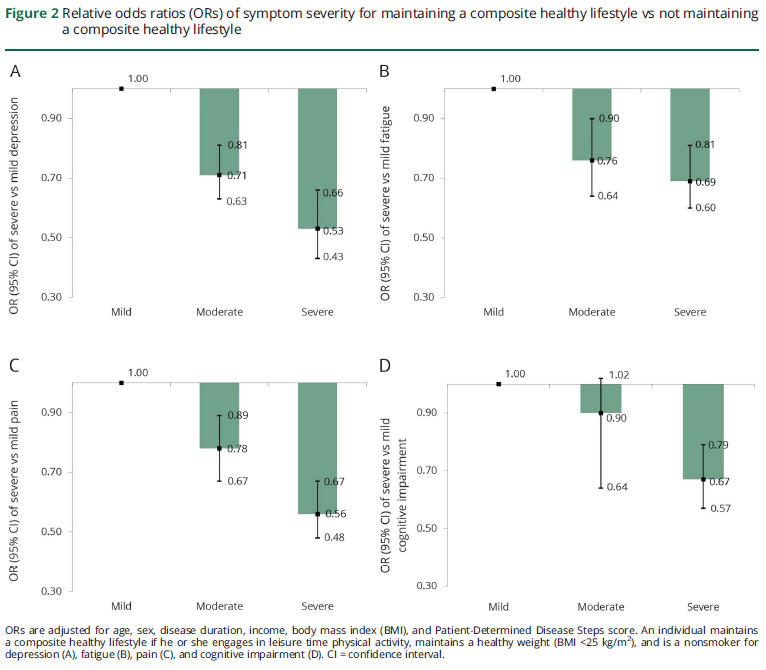
Any exposure to a specific diet or diet plan was inversely associated with increasing disability severity. Unexpectedly, adherence to the Wahls diet was associated with increasing disability severity. However, this diet is primarily targeted at patients with progressive disease, likely creating a selection bias of a specific subgroup of patients adhering to a particular diet, which may be driving some of the observed associations. Similarly, exposure to a gluten-free diet and lower intakes of dairy and calcium were also more common in patients with progressive vs relapsing-remitting disease. As a result, longitudinal assessment of people with MS who follow these diets is needed to better understand their effect on MS symptoms and evolution of disease course. Since smoking, obesity, insufficient physical activity, and inadequate nutrition contribute to most preventable causes of morbidity in the general population and have been heavily studied with respect to cardiovascular disease,25,26 we focused on these factors to derive our measure of a composite healthy lifestyle. Our findings are consistent with a smaller study of people with MS,34 which found that lifestyle factors including smoking, poor nutrition, excess alcohol intake, and no physical activity tended to co-occur, with more than half of the participants having more than one risk factor. The prevalence of severe depression, fatigue, pain, and cognitive symptoms was lower for our participants who maintained a composite healthier lifestyle than those who did not. While this cross-sectional study cannot determine whether a healthy lifestyle reduces MS symptoms or whether their severity hinders individuals from engaging in a fully healthy lifestyle, it provides a rationale for further study of the association’s directionality.
Our study has limitations. Respondents tended to be older, to be predominantly Caucasian, and to have longstanding MS. The association between diet and MS outcomes may differ in other subgroups of people with MS. However, the sample of participants in the registry appears representative of the general US MS population with respect to sex, age at symptom onset, and MS course.35 The DSQ lacked detailed information concerning potentially important dietary factors such as specific types of fats and could not differentiate between types of dairy foods.36,37 Further, our dietary score equally weights each component with respect to disability and symptoms, but different dietary components may contribute differentially to disease processes. Our study cannot determine whether the observed associations are disease-specific; adherence to a Mediterranean-style diet is associated with less brain atrophy in populations of older, otherwise healthy individuals.38 As noted, the design limits causal inference.
Our findings suggest that high-quality diets emphasizing intake of fruits, vegetables and legumes, and whole grains and low intakes of sugar and redmeat are associated with lower levels of disability in people with MS. Individuals who maintain a healthier lifestyle have a lower prevalence of severe depression, pain, fatigue, and cognitive problems. As diet and other lifestyle factors are modifiable, they offer a promising, safe avenue to ameliorate MS-associated symptoms and influence disease course. Longitudinal studies are needed to gain a better understanding of the directionality of the association between diet, a composite healthy lifestyle, and disease outcomes.
Author contributions
Kathryn C. Fitzgerald: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Tuula Tyry: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. Amber R. Salter: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, acquisition of data. Stacey S. Cofield: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. Robert J. Fox: drafting/ revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, study supervision. Gary R. Cutter: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Ruth Ann Marrie: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision.
Acknowledgment
Performance Scales Questions 9–16, Copyright Registration Number/Date: TXu000743629/1996-04-04; assigned to DeltaQuest Foundation, Inc., effective October 1, 2005. US
Copyright law governs terms of use.
Study funding
NARCOMS is supported in part by the Consortium of Multiple Sclerosis Centers (CMSC) and The Foundation of the CMSC. This study was supported by a fellowship grant to K.C.F. from the CMSC’s NARCOMS postdoctoral fellowship award.
Disclosure
K. Fitzgerald receives research funding from the Consortium of MS Centers and the National MS Society and in the form of postdoctoral fellowships. T. Tyry and A. Salter report no disclosures relevant to the manuscript. S. Cofield has received personal compensation for activities with Ortho Tech Biotech, the American Academy for Orthopedic Surgery, Oxford University Press, Department of Defense, and Medimmune. R. Fox receives consultant fees from Actelion, Biogen, Genentech, Novartis, and Teva. He has served on advisory committees for Biogen Idec and Novartis. He also receives research support from Biogen (clinical trial contracts) and Novartis (research study support). G. Cutter serves on Data and Safety Monitoring Boards for AMO Pharmaceuticals, Apotek, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck, Merck/Pfizer, Opko Biologics, Neurim, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee). He also serves on consulting or advisory boards for Atara Biotherapeutics, bioeq GmbH, CereSpir Inc., Consortium of MS Centers (grant), Genzyme, Genentech, Innate Therapeutics, Janssen Pharmaceuticals, Klein Buendel Incorporated, Medimmune, Medday, Nivalis, Novartis, Opexa Therapeutics, Roche, Savara Inc., Somahlution, Teva Pharmaceuticals, Transparency Life ciences, and TG Therapeutics. R. Marrie has conducted clinical trials for Sanofi-Aventis and receives research funding from CIHR, the National MS Society, the MS Society of Canada, the MS Scientific Research Foundation, Research Manitoba, the Consortium of MS Centers, Crohn’s and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis. Go to Neurology.org/N for full disclosures.
References
1. Oh J, O’Connor PW. Multiple sclerosis in 2014: progress in MS: classification,mechanisms and treatment. Nat Rev Neurol 2015;11:76–78.
2. Frohman EM, Racke MK, Raine CS. Multiple sclerosis: the plaque and its pathogenesis.N Engl J Med 2006;354:942–955.
3. von Geldern G, Mowry EM. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat Rev Neurol 2012;8:678–689.
4. Bisht B, Darling WG, Grossmann RE, et al. A multimodal intervention for patients with secondary progressive multiple sclerosis: feasibility and effect on fatigue. J Altern Complement Med 2014;20:347–355.
5. Wilmot VA, Swank RL. The influence of low-fat diet on blood lipid levels in health and in multiple sclerosis. Am J Med Sci 1952;223:25–34.
6. Veronese N, Stubbs B, Noale M, Solmi M, Luchini C, Maggi S. Adherence to the Mediterranean diet is associated with better quality of life: data from the Osteoarthritis Initiative. Am J Clin Nutr 2016;104:1403–1409.
7. Koch M, Jensen MK. Association of the MIND diet with cognition and risk of Alzheimer’s disease. Curr Opin Lipidol 2016;27:303–304.
8. Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of the NARCOMS registry: diagnosis. Mult Scler 2007;13:770–775.
9. Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient-Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013;13:37.
10. Schwartz CE, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS: North American Research Consortium on Multiple Sclerosis Outcomes Study Group. Neurology 1999;52:63–70.
11. Chamot E, Kister I, Cutter GR. Item response theory-based measure of global disability in multiple sclerosis derived from the Performance Scales and related items. BMC Neurol 2014;14:192.
12. Marrie RA, Goldman M. Validity of Performance Scales for disability assessment in multiple sclerosis. Mult Scler 2007;13:1176–1182.
13. Marrie RA, Goldman M. Validation of the NARCOMS registry: Tremor and Coordination Scale. Int J MS Care 2011;13:114–120.
14. Marrie RA, Cutter G, Tyry T, Hadjimichael O, Vollmer T. Validation of the NARCOMS Registry: pain assessment. Mult Scler 2005;11:338–342.
15. Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of NARCOMS depression scale. Int J MS Care 2008;10:81–84.
16. Marrie RA, Cutter G, Tyry T, Hadjimichael O, Campagnolo D, Vollmer T. Validation of the NARCOMS registry: fatigue assessment. Mult Scler 2005;11:583–584.
17. Dietary Screener Questionnaire (DSQ) in the NHANES 2009-10: Dietary Factors, Food Items Asked, and Testing Status for DSQ [Internet]. Available at: epi.grants.cancer.gov/nhanes/dietscreen/evaluation.html. Accessed March 2, 2017.
18. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–524.
19. Zong G, Gao A, Hu FB, Sun Q. Whole grain intake and mortality from all causes,cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation 2016;133:2370–2380.
20. Schwingshackl L, Schwedhelm C,Hoffmann G, et al. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2017;105:1462–1473.
21. Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer.Am J Clin Nutr 2010;92:1429–1435.
22. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720.
23. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–1100.
24. Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a dietary approaches to stop hypertension (DASH)–style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost 2012;10:189–198.
25. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613.
26. Gooding HC, Shay CM, Ning H, et al. Optimal lifestyle components in young adulthood are associated with maintaining the ideal cardiovascular health profile into middle age. J Am Heart Assoc 2015;4:e002048.
27. Briggs FB, Gunzler DD, Ontaneda D, Marrie RA. Smokers with MS have greater decrements in quality of life and disability than non-smokers. Mult Scler Epub 2017 Jan 1.
28. Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci 2015;18:125–136.
29. Leong EM, Semple SJ, Angley M, SiebertW, Petkov J,McKinnon RA. Complementary and alternative medicines and dietary interventions in multiple sclerosis: what is being used in South Australia and why? Complement Ther Med 2009;17:216–223.
30. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome inmultiple sclerosis. Nat Commun 2016;7:12015.
31. Dai J, Jones DP, Goldberg J, et al. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 2008;88:1364–1370.
32. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014;40:833–842.
33. Yadav V, Marracci G, Kim E, et al. Low-fat, plant-based diet in multiple sclerosis:a randomized controlled trial. Mult Scler Relat Disord 2016;9:80–90.
34. Balto JM, Ensari I, Hubbard EA, Khan N, Barnes JL, Motl RW. Individual and cooccurring SNAP risk factors. Int J MS Care 2016;18:298–304.
35. What is MS? [Internet.] National Multiple Sclerosis Society. Available at: nationalmssociety. org/What-is-MS. Accessed March 7, 2017.
36. Bjørnevik K, Chitnis T, Ascherio A, Munger KL. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult Scler Epub 2017 Jan 1.
37. Haghikia A, J¨org S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015;43:817–829.
38. Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015;85:1744–1751.