Pregnancy in the Setting of Multiple Sclerosis
Original Article
Pregnancy in the Setting of Multiple Sclerosis
CONTINUUM
Michelle Fabian, MD
ABSTRACT
Purpose of Review: This article provides a review of the available data on reproductive issues that arise in patients with multiple sclerosis (MS).
Recent Findings: Recent findings have replicated earlier findings that pregnancy and possibly breast-feeding bring about a favorable immunomodulatory effect in patients with MS. Use of disease-modifying therapies prior to pregnancy may further decrease a patient’s risk for postpartum disease activity.
Summary: The annualized relapse rate in MS decreases during pregnancy, with a nadir in the third trimester, and rebounds significantly in the 3-month postpartum period. Exclusive breast-feeding may exert a beneficial effect in decreasing the postpartum risk for relapse. Certain assisted reproductive technology methods are thought to increase the risk for relapse. Disease-modifying therapies are generally discontinued during pregnancy and lactation with a few exceptions. The pregnancy course is usually routine without significant obstetric complications, and babies, although slightly smaller, are typically healthy.
INTRODUCTION
Women are most often diagnosed with multiple sclerosis (MS) during their childbearing years. Because of the coincident timing, it is essential that patients are educated about the available data surrounding MS and reproductive issues. It is wise to introduce the topic early after the diagnosis of MS is made, both to educate the patient and to gain an understanding about the patient’s concerns and plans. The topic should then be touched upon briefly at subsequent visits. For many patients, a dedicated visit at some point to discuss these issues is extremely beneficial. The discussion should broadly be structured around three key areas: the effects of pregnancy and other hormonal alterations on the MS course, evidence regarding disease-modifying therapies and pregnancy, and the effects of MS on the pregnancy course. This review covers these areas in detail.
PREGNANCY AND RELATED HORMONAL ALTERATIONS AND THE MULTIPLE SCLEROSIS COURSE
Pregnancy, breast-feeding, fertility treatments, and oral contraceptives all produce changes to the hormonal milieu, which may impact the MS course in a patient to varying degrees. The effects can be favorable or adverse depending on the alteration that takes place.
Pregnancy
Up until the late 20th century, many neurologists advised patients with MS to avoid pregnancy, as it was thought to worsen the MS course. A paradigm shift occurred in 1998 when Confavreux and colleagues1 published the Pregnancy in Multiple Sclerosis (PRIMS) study. PRIMS was the first large prospective study aimed at understanding the influence of pregnancy on the annualized relapse rate and risk for progression in MS. The study followed 254 women with relapsing-remitting MS prospectively during pregnancy and the postpartum/breast-feeding period and compared the annualized relapse rate to the year prior to conception, using the patient as her own control.
Most important, the PRIMS study demonstrated a decreased annualized relapse rate during pregnancy, with a statistically significant nadir in the third trimester (annualized relapse rate 0.2 T 1.0; mean T SD), compared with the year before pregnancy (annualized relapse rate 0.7 T 0.9). Additionally, in the 3 months postpartum, the rate of relapse significantly increased (1.2 T 2.0), although only 28% of the cohort actually experienced a postpartum relapse (Figure 8-1). The annualized relapse rate from postpartum months 3 through 12 was not significantly different compared to the prepregnancy year. When the annualized relapse rate for the 12-month combined period of pregnancy and 3-month postpartum period was compared to the annualized relapse rate for the year prior to pregnancy, no significant difference was shown. Disability accumulation did not occur at a more rapid rate postdelivery than prior to pregnancy. A follow-up study to PRIMS looked at the factors that predicted a relapse. This study found the risk for postpartum relapse was greatest in those who had experienced a relapse in the year prior to pregnancy or during pregnancy, and the risk was also weakly correlated to number of years from diagnosis.2 The findings of PRIMS have been replicated multiple times since,3Y5 although the relapse rates and magnitude of postpartum rebound have generally been found to be less than that of PRIMS. Interestingly, a large international cohort of pregnant patients found that use of a disease-modifying therapy at some point in the 2 years prior to pregnancy provided a 45% lower risk for postpartum relapse, regardless of preconception relapse rate.6
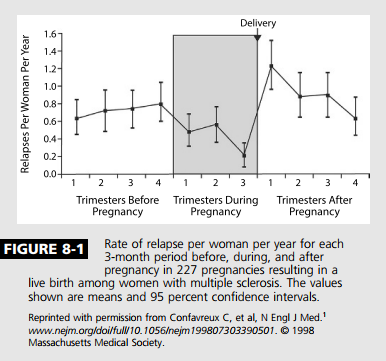
The fluctuation in relapse rate during pregnancy and beyond likely happens as a byproduct of the hormonally mediated immune shift that occurs in pregnancy away from a more inflammatory TH1/TH2 T-cell profile toward a more protective TH2/TH1 profile. In doing so, the immune system is better able to manage the “foreign” genetic material of the fetus and protects both fetus and mother. This important immunomodulatory shift, although only one of a multitude of cellular and cytokine shifts that occur, may begin to explain why MS and other cellmediated conditions such as rheumatoid arthritis are seen to improve during pregnancy while antibodymediated conditions such as systemic lupus erythematosus and neuromyelitis optica (NMO) often worsen.7
Long-term modulation of disease course with pregnancy. Although it has been well established that pregnancy has an immunomodulatory effect on the peripartum and postpartum period in MS, perhaps even more interesting, some evidence exists that pregnancy produces a decreased risk for developing MS and brings about relative attenuation of the long-term MS disease course as well. Runmarker and Anderson8 found a decreased risk for a diagnosis of MS in parous women and also a decreased risk for development of progressive disease in women who became pregnant after an MS diagnosis. Other studies found that having a child after diagnosis of MS increased the time to use of wheelchair9 and time to an Expanded Disability Status Scale (EDSS) score of 6.0.10 The implications of these findings have been debated, as a diagnosis of MS may alter a woman’s decision to have children. The Australian Multi-centre Study of Environment and Immune Function (Ausimmune) attempted to address this issue by studying women who developed a first demyelinating event only after they had had children. This study found that the risk of a first demyelinating event was inversely correlated to number of children, with the lowest risk in women with four or more children.11 Hence, just as pregnancy has been shown to alter MS disease activity in the short term, it may favorably impact the long-term disease course as well. Although a mechanism for this effect is not known, some have hypothesized that an exchange of hematopoietic stem cells between mother and fetus may play a role.11
Breast-feeding
Making the decision of whether or not to breast-feed may be equally or more challenging for a patient with MS than the decision of getting pregnant. On the one hand, obstetricians and pediatricians advise “breast is best.” On the other hand, patients and their doctors often have real concerns about the return of disease activity, and diseasemodifying therapies are generally not advised while breast-feeding.
Because it is extremely unlikely that a randomized trial that would definitively answer this question could be conducted, the true effect of breastfeeding on annualized relapse rate is still debated. Taking the limitations into account, studies have found either a neutral1,4,12 or beneficial13,14 effect on annualized relapse rate in women who breast-feed. Importantly, an effect may have been missed in the studies that did not separate those who exclusively breast-fed from those who supplemented with formula.15
A meta-analysis of 12 studies included a combined total of 869 patients who breast-fed versus 689 who did not. It demonstrated a 47% decrease in postpartum annualized relapse rate for those women who breast-fed. The authors acknowledged that the study was limited by the heterogeneity of the groups, with differences including amount of breast-feeding (exclusive versus not), prepregnancy MS activity, and disease-modifying therapy use.16
Although the question may never be fully answered, the available evidence suggests that breast-feeding is safe and possibly even beneficial for the patient with MS. Therefore, although each case should be considered uniquely, the choice to breast-feed should generally be encouraged and supported.
Assisted Reproductive Technology
Patients with MS are generally assumed to have the same fertility rate as the general population, although a 2015 study of patients with MS found significantly lower levels of antimu¨llerian hormone, a hormone known to correlate with ovarian reserve,17 and the use of assisted reproductive technology is likely increased in patients with MS.18 Reasons for this include delay of childbearing in the context of an MS diagnosis, high rates of sexual dysfunction, increased rates of endocrinologic and hormonal disorders, and increased rates of endometriosis.19
No large studies exist of the effect of assisted reproductive technology on MS to date. However, a number of small case series have been published. They suggest that an increased risk of relapse exists with the use of certain forms of assisted reproductive technology. Two factors that seem to increase the risk for relapse appear to be an unsuccessful cycle of assisted reproductive technology20 and the use of a gonadotropin-releasing hormone (GnRH) agonist.21,22 The only prospective series on assisted reproductive technology in patients with MS found a sevenfold increased risk for relapse after receiving treatment with a GnRH agonist.23 Therefore, although the evidence is limited at present, fertility specialists might be advised to use a protocol that avoids the use of a GnRH agonist, if possible, in patients with MS who require assisted reproductive technology.
Oral Contraceptives
A few studies have looked at the impact of oral contraceptives on the risk for MS and on disease course. Two studies have found the incidence of MS to be less with use of oral contraceptives,24,25 contrary to the findings of two older studies in which the effect was neutral.26,27 A few studies also found lower disability among patients who had used oral contraceptives compared with those patients who had not.28,29 No published data suggest that oral contraceptives interact in a negative manner with disease-modifying therapies, and a small randomized trial of interferon beta and oral contraceptives suggested a possible benefit as a combination therapy, with a decreased annualized relapse rate and less MRI activity.30 Of course, it is important to check interactions between oral contraceptives and other medications that might be given to a patient with MS, such as steroids or antibiotics.
Use of Disease-Modifying Therapies During Pregnancy and Lactation
One of the key reasons for continued reproductive counseling of female patients with MS is to fully educate them about the potential risks of conceiving while on a disease-modifying therapy (Table 8-1). The clinician should never assume that a patient knows the risks associated with a drug, even if discussed previously. A patient may be preoccupied with the diagnosis or, over time, may forget initial warnings. Thus, reinforcement of the need for contraception while on a diseasemodifying therapy is critical. Some additional considerations regarding disease-modifying therapy discontinuation in the setting of pregnancy can be found in the article “Switching or Discontinuing Disease-Modifying Therapies for Multiple Sclerosis” by Aaron E. Miller, MD, FAAN,31 in this issue of Continuum.
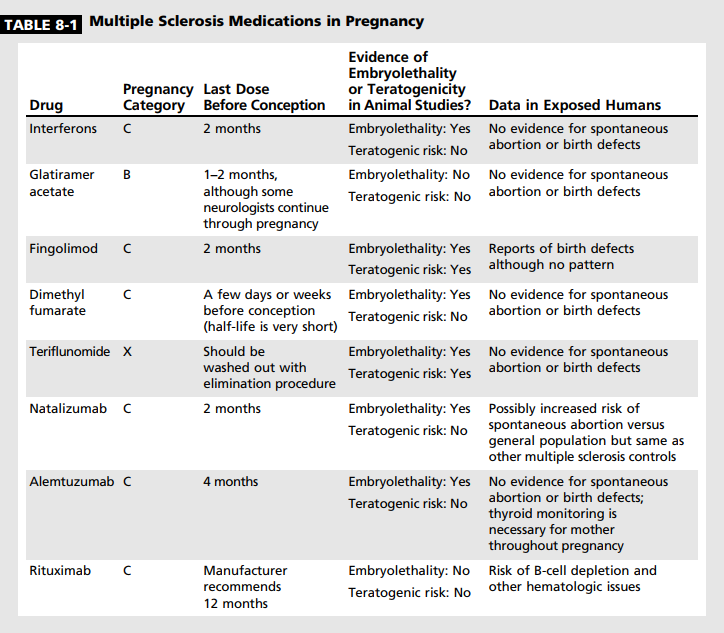
Injectables. As interferon beta is the oldest disease-modifying therapy, pregnancy data are the most plentiful for it among all of the MS drugs. Monkeys appeared to have an increased rate of spontaneous abortion when given interferon beta at 40 times the recommended dose,32 although an increased miscarriage rate has not been seen in humans. No evidence exists of an increased risk of congenital malformations.33 However, because of the animal data regarding spontaneous abortions, it is typically recommended that interferons be discontinued 1 to 2 months before conception.
Glatiramer acetate has the most favorable historical data regarding pregnancy. No evidence exists for glatiramer acetate causing fertility issues, miscarriage, or congenital malformations in the 500 cases reported in the literature.34 Because of this, some neurologists will allow their patients to continue on glatiramer acetate during conception and pregnancy.
Both glatiramer acetate and interferon beta are large molecules that are not likely to be transmitted into breast milk in large quantities and thus are likely safe for use during lactation. Although no human studies have been published regarding glatiramer acetate, a small study was done with interferon beta that showed the drug level in breast milk to be 0.006% that of the maternal dose with no side effects to the baby.35
Oral medications. The oral diseasemodifying therapies for MS include fingolimod, dimethyl fumarate, and teriflunomide.
Fingolimod. Fingolimod does not appear to reduce fertility. Animal studies of fingolimod showed evidence for teratogenicity and embryolethality at doses lower than the therapeutic human dose. The most common malformations were persistent truncus arteriosus and ventricular septal defect.36 Results from a pregnancy registry have been published outlining the outcomes of 66 pregnancies. A slightly higher rate of spontaneous abortion was found, and multiple malformations were reported, including acrania, a malformation of the tibia, and tetralogy of Fallot.37 Fingolimod should be stopped 2 months before conception. It is excreted into animal breast milk and is orally bioavailable; thus, it should not be used during breast-feeding.
Dimethyl fumarate. Fertility does not appear to be affected by dimethyl fumarate. The half-life of dimethyl fumarate is extremely short, and thus, although it should not be continued after conception, it may be stopped shortly beforehand. Evidence from animal studies indicates that dimethyl fumarate crosses the placenta. The highest dose tested showed an increased rate of spontaneous abortion as well as lower fetal weight and delayed ossification. Administration of dimethyl fumarate to rats throughout organogenesis and lactation was associated with delayed sexual maturation and reduced testicular weight at the highest dose tested. Neurobehavioral impairment was observed at all doses.38 A case series of 45 women exposed to dimethyl fumarate during early pregnancy has been reported. In this group of women, no evidence was shown for effect on babies that were born.39 No information is available concerning dimethyl fumarate and human breast milk, and it should be avoided during lactation.
Teriflunomide. Teriflunomide is contraindicated in pregnancy owing to animal studies that demonstrated embryotoxicity and teratogenicity at recommended human doses. If a patient on teriflunomide decides to become pregnant, or becomes pregnant accidentally, she must undergo elimination with cholestyramine. Despite the pregnancy contraindication, 70 pregnancies with exposure to teriflunomide have been reported from clinical trials.40 The rate of spontaneous abortion was not different from the general population, and no serious malformations were reported. Teriflunomide has no known effect on male fertility, although animal studies have demonstrated reduced sperm counts. It is believed that maleto-female semen transfer results in a detectable female plasma exposure to teriflunomide at a level 100 times lower than the therapeutic dose.41 Of 19 pregnancies fathered by male patients on teriflunomide, none showed any evidence of malformation or increased rate of miscarriage.40 Nonetheless, males wishing to father a child should also consider undergoing elimination. Animal studies have found teriflunomide in breast milk; therefore, teriflunomide is contraindicated for breast-feeding.41
Monoclonal antibodies. Monoclonal antibodies are another form of diseasemodifying therapy for the treatment of MS.
Natalizuma b. Supratherapeutic doses of natalizumab have been shown to decrease fertility in animal models, although not permanently. Decreased fertility has not been shown in humans. In animal studies, natalizumab given at supratherapeutic doses have demonstrated reduced survival, anemia, and thrombocytopenia in newborn offspring.42 The Tysabri Pregnancy Exposure Registry reported 375 pregnancies that resulted in 314 live births. The rates of miscarriage and malformation were not increased versus the general population.43 Ebrahimi and colleagues44 reported data from 102 patients with MS exposed to natalizumab at some point in pregnancy compared to a group of controls with MS and a group of healthy controls. In this cohort, both patient groups with MS had a higher rate of miscarriage, lower birth weight, and shorter birth length than the healthy controls. The rate of malformations was not elevated above the expected rate.44 Also, a case series reported 13 patients who received natalizumab in the third trimester only. Of these babies, 10 of 13 showed evidence for mild hematologic abnormalities but overall were healthy.45 It is recommended that natalizumab should be discontinued 2 months prior to conception.42 Natalizumab is transferred into human breast milk, yet it is not orally bioavailable. Nonetheless, it should not be given during breast-feeding.
Alemtuzuma b. Alemtuzumab given to mice during organogenesis resulted in increased rates of fetal loss as well as decreased B- and T-lymphocyte populations at birth. Patients should avoid conception for 4 months after alemtuzumab infusion. Thyroid dysfunction can develop months or years after dosing; thus, thyroid function should be monitored closely as thyroid disorders can significantly affect fetal wellbeing.46 In a 2014 study of 104 patients previously dosed with alemtuzumab, 139 pregnancies were reported. All but six of these pregnancies were conceived at least 4 months after the mother had received an alemtuzumab infusion. In this series of patients, no indication of increased rates of miscarriage or malformations was observed. One case of neonatal thyrotoxic crisis occurred and resolved with appropriate treatment.47 Alemtuzumab is present in the milk of lactating mice; no human lactation data are available. Similar to pregnancy, breast-feeding should be avoided within 4 months of alemtuzumab dosing.
Rituxima b. Rituximab has been used more frequently in recent years to treat MS, and, if approved, the humanized analogue ocrelizumab will likely also be utilized. Rituximab crosses the placenta in animal studies, and although no evidence of increased risk of miscarriage or teratogenicity was shown in these studies, consistent B-cell depletion was shown in the newborn.48 In humans, rituximab has been detected at low levels in the blood up to 24 weeks after infusion,49 and it is recommended that conception be delayed 12 months after the last infusion, although this may be a longer time period than necessary. Outcomes from 153 pregnancies with rituximab exposure were published by Chakravarty and colleagues50 in 2011. Twenty-four percent of babies were born prematurely between 30 and 37 weeks, a rate that is higher than in the general population. No signals of increased risk for teratogenicity were observed in this cohort. Eleven babies were reported to have hematologic abnormalities, including B-cell depletion, thrombocytopenia, granulocytopenia, and anemia, although some mothers were dosed with multiple immunosuppressants concurrently. No infections were reported in the babies with B-cell depletion; however, one baby had a cerebral hemorrhage secondary to thrombocytopenia.50 The risk of B-cell depletion in the newborn appears to be higher when the mother is dosed in the second or third trimester. Rituximab is found in the breast milk of lactating monkeys in low amounts, and, because data are insufficient in humans, breast-feeding is not advised.
Chemotherapeutics. Mitoxantrone and cyclophosphamide are chemotherapeutics used less commonly in the modern disease-modifying therapy era. Both mitoxantrone and cyclophosphamide have significant effects on fertility, causing amenorrhea in up to 26% and 33% of women, respectively. Decreased fertility is common in men as well, although the effect may be more reversible than in women.51 Both agents have been found to be embryotoxic in animal studies, and their use should be avoided in pregnancy. Likewise, both drugs have been found to be present in human breast milk, and thus breast-feeding should be avoided.
TREATMENT AND PREVENTION OF RELAPSE DURING PREGNANCY, POSTPARTUM, AND LACTATION
Despite the significant reduction in MS activity during pregnancy, the fact remains that relapses during pregnancy still do occur for some patients. The treatment of an MS relapse in a pregnant patient should be approached in a similar, although perhaps more conservative, manner as other MS relapses. Urinary tract infections, both symptomatic and asymptomatic, are more common in pregnancy and should be ruled out as a cause of pseudorelapse. MRI without gadolinium is likely safe52 although usually not necessary to decide upon a relapse management plan. Gadolinium should be avoided unless it is critically necessary. Although it has never been linked to malformations or pregnancy complications, gadolinium has been shown to immediately cross the placenta in primate studies; it is unknown if this could be of consequence to a fetus. Less than 0.1% of the gadolinium dose is thought to enter the breast milk. Therefore, it is likely safe to continue breast-feeding after receiving contrast, although interrupting breastfeeding for 24 hours is a conservative approach favored by some patients and physicians.53
The use of steroids should be avoided in the first trimester if at all possible owing to data from multiple studies that suggest that there is a small but increased risk for cleft palate during this time,54 although more recent studies could not find evidence for an increased risk.55 In the second and third trimesters, despite a potential risk for preterm labor and lower birth weight,56 prednisone, prednisolone, and methylprednisolone are thought to be safe to the fetus as minimal transfer of these drugs across the placenta occurs.57 Steroids transfer minimally into breast milk. If a breastfeeding mother requires steroids, it is recommended that she pump and discard the milk for 4 hours after each treatment (Case 8-1).58 Thus, the standard MS relapse treatment regimen of 1000 mg/d IV for 3 to 5 days may be employed.

Prevention of Postpartum Relapse
Multiple small trials have looked at ways to reduce the risk of a postpartum relapse, although no definitive evidence exists that any option is truly effective in doing so. In 2004, Achiron and colleagues59 reported a retrospective study of women who received IV immunoglobulin (IVIg) during pregnancy and postpartum versus only postpartum versus a control group. The group that received IVIg during pregnancy and beyond had the lowest relapse rate. Haas and colleagues60 conducted a prospective randomized study of high-dose versus low-dose IVIg within 24 hours of delivery, with both groups then receiving five equivalent monthly infusions. The study showed no evidence for a dose effect on the relapse rate, calling into question the efficacy of this approach.
Prophylactic treatment with steroids has also been studied in a limited fashion. De Seze and colleagues61 gave 20 women 1 g IV methylprednisolone monthly for 6 months and found a decreased postpartum relapse rate compared to a historical control group. A retrospective nonrandomized series of 52 pregnancies found that 39 patients who were given a single dose of 1000 mg IV methylprednisolone at the time of delivery had a decrease in relapse rate in the first 3 months postpartum compared to 13 patients who had no treatment (18% versus 46% [P=.04]).62
Hormonal therapy was studied as a third preventive strategy in the Prevention of Post Partum Relapses With Progestin and Estradiol in Multiple Sclerosis (POPARTMUS) trial. This study randomly assigned postpartum women into a progestin/estriol arm versus a placebo arm. Although the trial was stopped early because of difficulty with enrollment, no difference in relapse rates was shown between treatment arms in the 202 women who were studied.63
Currently, because of a lack of definitive data, strategies used to prevent postpartum relapses usually depend on two factors: (1) the patient’s desire to breast-feed and (2) the level of concern for a severe postpartum course because of previous disease activity. The breastfeeding patient will typically continue off disease-modifying therapy (except in the case of a patient on glatiramer acetate or interferon as discussed earlier) until weaning. For the nonY breast-feeding patient, a diseasemodifying therapy should be started as soon as is feasible after delivery.
The Effect of Multiple Sclerosis on the Pregnancy Course
The patient with MS considering pregnancy may have significant concerns about the pregnancy course from an obstetrics standpoint and about risks to the child. Dahl and colleagues64 found increased rates of operative deliveries (forceps, vacuum, or cesarean delivery) in mothers with MS versus controls as well as decreased birth weight and length in babies born to mothers with MS. Apgar scores, mortality, and incidence of birth defects did not differ between the two groups. Kelly and colleagues65 used a US national database and compared patients with MS, patients with diabetes mellitus, and patients with epilepsy with healthy controls. They found that patients with MS did not have a higher rate of premature birth but did have a slightly increased risk for gestational hypertension (10.7% versus 8.5%), intrauterine growth restriction (2.7% versus 1.9%), and rate of cesarean delivery (42.4% versus 38%). Yet another database study from British Columbia did not find a difference between gestational age at delivery or Apgar scores in children born to mothers with MS, yet did find a slightly increased rate of assisted vaginal delivery or cesarean delivery in mothers with MS versus controls. In this study, mean birth weight did not differ from healthy controls.66
Evidence supports the use of epidural and spinal anesthesia as safe options. The PRIMS study did not find epidural anesthesia to be a risk factor for postpartum relapse.1 A 2012 study of Italian patients with MS also found no correlation between epidural anesthesia or cesarean delivery with relapse or worsening of the EDSS in the postpartum period.67
Thus, pregnancies in patients with MS are generally well tolerated, and children have slightly decreased birth weights but are equally healthy. Rates of cesarean delivery appear to be slightly higher in patients with MS. No evidence exists for epidural anesthesia causing worsening of MS, and it should be used if the patient desires. From the obstetrics standpoint, patients should be cared for like mothers without MS, with no need for special precautions unless dictated by other maternal or fetal issues.
CONCLUSION
In the past 2 decades, new evidence has led to a reversal in thinking surrounding MS and pregnancy. Overall, the data suggest that pregnancy is a beneficial state for the patient with MS, both in the short and the long term, and that the children born to mothers with MS are typically healthy. Women who wish to become pregnant should be counseled regarding the benefits and risks regarding pregnancy and the postpartum period. Prevention of fetal exposure to diseasemodifying therapies by thorough counseling is critical.
The doctor-patient relationship in MS is multifaceted. Issues surrounding pregnancy often factor heavily into management decisions. Taking a proactive role in informing and supporting patients regarding reproductive decisions they may face is essential as it is part of the comprehensive approach to MS care.
REFERENCES
1. Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339(5):285Y291. doi:10.1056/ NEJM199807303390501.
2. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 2004; 127(pt 6):1353Y1360. doi:10.1093/brain/ awh152.
3. Salemi G, Callari G, Gammino M, et al. The relapse rate of multiple sclerosis changes during pregnancy: a cohort study. Acta Neurol Scand 2004;110(1):23Y26. doi:10.1111/j.1600-0404.2004.00270.x.
4. Portaccio E, Ghezzi A, Hakiki B, et al. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 2011;77(2):145Y150. doi:10.1212/ WNL.0b013e318224afc9.
5. Fragoso YD, Finkelsztejn A, Comini-Frota ER, et al. Pregnancy and multiple sclerosis: the initial results from a Brazilian database. Arq Neuropsiquiatr 2009;67(3A):657Y660. doi:10.1590/ S0004-282X2009000400015.
6. Hughes SE, Spelman T, Gray OM, et al. Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler 2014;20(6):739Y746. doi:10.1177/ 1352458513507816.
7. Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol 2012;8(5): 255Y263. doi:10.1038/nrneurol.2012.43.
8. Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain 1995;118(pt 1):253Y261. doi:10.1093/ brain/118.1.253.
9. Verdru P, Theys P, D’Hooghe MB, Carton H. Pregnancy and multiple sclerosis: the influence on long term disability. Clin Neurol Neurosurg 1994;96(1):38Y41. doi:10.1016/0303-8467(94)90027-2.
10. D’Hooghe MB, Nagels G, Uitdehaag BM. Long-term effects of childbirth in MS. J Neurol Neurosurg Psychiatry 2010;81(1):38Y41. doi:10.1136/ jnnp.2008.163816.
11. Ponsonby AL, Lucas RM, van der Mei IA, et al. Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology 2012;78(12):867Y874. doi:10.1212/ WNL.0b013e31824c4648.
12. Nelson LM, Franklin GM, Jones MC. Risk of multiple sclerosis exacerbation during pregnancy and breast-feeding. JAMA 1988;259(23):3441Y3443. doi:10.1001/ jama.1988.03720230051029.
13. Langer-Gould A, Huang SM, Gupta R, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol 2009;66(8):958Y963. doi:10.1001/archneurol.2009.132.
14. Hellwig K, Haghikia A, Rockhoff M, Gold R. Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther Adv Neurol Disord 2012;5(5):247Y253. doi:10.1177/1756285612453192.
15. Bove R, Alwan S, Friedman JM, et al. Management of multiple sclerosis during pregnancy and the reproductive years: a systematic review. Obstet Gynecol 2014;124(6):1157Y1168. doi:10.1097/ AOG.0000000000000541.
16. Pakpoor J, Disanto G, Lacey MV, et al. Breastfeeding and multiple sclerosis relapses: a meta-analysis. J Neurol 2012;259(10): 2246Y2248. doi:10.1007/s00415-012-6553-z.
17. Tho¨ ne J, Kollar S, Nousome D, et al. Serum anti-Mu¨ llerian hormone levels in reproductive-age women with relapsing-remitting multiple sclerosis. Mult Scler 2015;21(1):41Y47. doi:10.1177/ 1352458514540843.
18. Jalkanen A, Alanen A, Airas L; Finnish Multiple Sclerosis and Pregnancy Study Group. Pregnancy outcome in women with multiple sclerosis: results from a prospective nationwide study in Finland. Mult Scler 2010;16(8):950Y955. doi:10.1177/ 1352458510372629.
19. Cavalla P, Rovei V, Masera S, et al. Fertility in patients with multiple sclerosis: current knowledge and future perspectives. Neurol Sci 2006;27(4):231Y239. doi:10.1007/s10072-006-0676-x.
20. Hellwig K, Beste C, Brune N, et al. Increased MS relapse rate during assisted reproduction technique. J Neurol 2008;255(4):592Y593. doi:10.1007/s00415-008-0607-2.
21. Laplaud DA, Leray E, Barrie`re P, et al. Increase in multiple sclerosis relapse rate following in vitro fertilization. Neurology 2006;66(8):1280Y1281. doi:10.1212/ 01.wnl.0000208521.10685.a6.
22. Michel L, Foucher Y, Vukusic S, et al. Increased risk of multiple sclerosis relapse after in vitro fertilisation. J Neurol Neurosurg Psychiatry 2012;83(8):796Y802. doi:10.1136/jnnp-2012-302235.
23. Correale J, Farez MF, Ysrraelit MC. Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol 2012;72(5):682Y694. doi:10.1002/ ana.23745.
24. Alonso A, Jick SS, Olek MJ, et al. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol 2005; 62(9):1362Y1365. doi:10.1001/ archneur.62.9.1362.
25. Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril 2010;94(7):2835Y2837. doi:10.1016/ j.fertnstert.2010.06.045.
26. Herna´n MA, Hohol MJ, Olek MJ, et al. Oral contraceptives and the incidence of multiple sclerosis. Neurology 2000;55(6):848Y854. doi:10.1212/WNL.55.6.848.
27. Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of multiple sclerosis. Br J Obstet Gynaecol 1998;105(12):1296Y1299. doi:10.1111/ j.1471-0528.1998.tb10008.x.
28. Sena A, Couderc R, Vasconcelos JC, et al. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J Neurol Sci 2012;317(1Y2):47Y51. doi:10.1016/ j.jns.2012.02.033.
29. Gava G, Bartolomei I, Costantino A, et al. Long-term influence of combined oral contraceptive use on the clinical course of relapsing-remitting multiple sclerosis. Fertil Steril 2014;102(1):116Y122. doi:10.1016/ j.fertnstert.2014.03.054.
30. Pozzilli C, De Giglio L, Barletta VT, et al. Oral contraceptives combined with interferon " in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015;2(4):e120. doi:10.1212/ NXI.0000000000000120.
31. Miller AE. Switching or discontinuing disease-modifying therapies for multiple sclerosis. Continuum (Minneap Minn) 2016;(3 Multiple Sclerosis and Other Demyelinating Diseases);851Y863.
32. AVONEX (interferon beta-1a) intramuscular injection. Full Prescribing Information. www.avonex.com/pdfs/Avonex_ Prescribing_Information.pdf. Accessed March 31, 2016.
33. Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler 2011;17(4):423Y430. doi:10.1177/ 1352458510394610.
34. Fragoso YD. Glatiramer acetate to treat multiple sclerosis during pregnancy and lactation: a safety evaluation. Expert Opin Drug Saf 2014;13(12):1743Y1748. doi:10.1517/14740338.2014.955849.
35. Hale TW, Siddiqui AA, Baker TE. Transfer of interferon "-1a into human breastmilk. Breastfeed Med 2012;7(2):123Y125. doi:10.1089/bfm.2011.0044.
36. GILENYA (fingolimod) capsules, for oral use. Full Prescribing Information. www.pharma. us.novartis.com/product/pi/pdf/gilenya.pdf. Revised August 2015. Accessed March 31, 2016.
37. Karlsson G, Francis G, Koren G, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology 2014; 82(8):674Y680. doi:10.1212/ WNL.0000000000000137.
38. TECFIDERA (dimethyl fumarate) delayed-release capsules, for oral use. Full Prescribing Information. www.tecfidera.com/ pdfs/full-prescribing-information.pdf. Issued April 2015. Accessed March 31, 2016.
39. Gold R, Phillips J, Havrdova E, et al. Delayed-release dimethyl fumarate and pregnancy: preclinical studies and pregnancy outcomes from clinical trials and postmarketing experience. P839. Presented at: Presented at Joint American Committee For Treatment and Research in Multiple Sclerosis (ACTRIMS)-European Committee For Treatment and Research in Multiple Sclerosis (ECTRIMS) Meeting; September 10Y13, 2014; Boston, Massachusetts.
40. Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther 2014;3(2):133Y138. doi:10.1007/s40120-014-0020-y.
41. AUBAGIO (teriflunomide) tablets, for oral use. Full Prescribing Information. products.sanofi.us/aubagio/aubagio.pdf. Published October 2014. Accessed March 31, 2016.
42. TYSABRI (natalizumab) injection, for intravenous use. Full Prescribing Information. www.tysabri.com/prescribingInfo. Revised May 2015. Accessed March 31, 2016.
43. Cristiano L, Friend S, Bozic C, Bloomgren G. Evaluation of pregnancy outcomes from the TYSABRI (natalizumab) pregnancy exposure registry. P02.127. Poster presented at: 65th Annual Meeting of the American Academy of Neurology; March 16Y23, 2013; San Diego, California.
44. Ebrahimi N, Herbstritt S, Gold R, et al. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler 2015;21(2):198Y205. doi:10.1177/1352458514546790.
45. Haghikia A, Langer-Gould A, Rellensmann G, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol 2014;71(7):891Y895. doi:10.1001/ jamaneurol.2014.209.
46. LEMTRADA (alemtuzumab) injection, for intravenous use. Full Prescribing Information. products.sanofi.us/Lemtrada/Lemtrada.pdf. Issued November 2014. Accessed March 31, 2016.
47. McCombe P, Achiron A, Giovannoni G, et al. Pregnancy outcomes in the alemtuzumab multiple sclerosis clinical development program. Presented at Joint American Committee For Treatment and Research in Multiple Sclerosis (ACTRIMS)-European Committee For Treatment and Research in Multiple Sclerosis (ECTRIMS) Meeting; September 10Y13, 2014; Boston, Massachusetts.
48. RITUXANA (rituximab) injection, for intravenous use. Full Prescribing Information. www.gene.com/download/pdf/rituxan_ prescribing.pdf. Revised August 2014. Accessed March 31, 2016.
49. Thurlings RM, Teng O, Vos K, et al. Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2010;69(2):409Y412. doi:10.1136/ard.2009.109041.
50. Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011;117(5):1499Y1506. doi:10.1182/ blood-2010-07-295444.
51. Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2015;29(3):207Y220. doi:10.1007/ s40263-015-0238-y.
52. Tremblay E, The´rasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics 2012;32(3):897Y911. doi:10.1148/rg.323115120.
53. American College of Radiology Committee on Drugs and Contrast Media. Administration of contrast media to women who are breast-feeding. In: ACR manual on contrast media. Version 10.1. Reston, VA: American College of Radiology, 2015:99.
54. Carmichael SL, Shaw GM, Ma C, et al; National Birth Defects Prevention Study. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol 2007;197(6): 585.e1Y585.e7. doi:10.1016/j.ajog. 2007.05.046.
55. Skuladottir H, Wilcox A, McConnaughey R, et al. First-trimester nonsystemic corticosteroid use and the risk of oral clefts in Norway. Ann Epidemiol 2014;24(9):635Y640. doi:10.1016/ j.annepidem.2014.06.005.
56. Pirson Y, Van Lierde M, Ghysen J, et al. Retardation of fetal growth in patients receiving immunosuppressive therapy. N Engl J Med 1985;313(5):328. doi:10.1056/ NEJM198508013130516.
57. Blanford AT, Murphy BE. In vitro metabolism of prednisolone, dexamethasone, betamethasone, and cortisol by the human placenta. Am J Obstet Gynecol 1977;127(3):264Y267.
58. Hoes JN, Jacobs JW, Boers M, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 2007;66(12):1560Y1567. doi:10.1136/ ard.2007.072157.
59. Achiron A, Kishner I, Dolev M, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol 2004;251(9): 1133Y1137. doi:10.1007/s00415-004-0495-z.
60. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler 2007;13(7):900Y908. doi:10.1177/ 1352458506075654.
61. de Seze J, Chapelotte M, Delalande S, et al. Intravenous corticosteroids in the postpartum period for reduction of acute exacerbations in multiple sclerosis. Mult Scler 2004;10(5):596Y597. doi:10.1191/ 1352458504ms1079sr.
62. Avila-Ornelas J, Avila M, Stosic M, et al. The role of postpartum intravenous corticosteroids in the prevention of relapses in multiple sclerosis. Int J MS Care 2011; 13(2):91Y93. doi:10.7224/1537-2073-13.2.91.
63. Vukusic S, El-Etr M, Ionescu I, et al; POPARTMUS Investigators Group. The POPARTMUS French-Italian multicentric trial of Post Partum Progestin and Estriol in Multiple Sclerosis: final results. Presented at: 28th Congress of the European Committee For Treatment and Research in Multiple Sclerosis; October 12, 2012; Lyon, France.
64. Dahl J, Myhr KM, Daltveit AK, et al. Pregnancy, delivery, and birth outcome in women with multiple sclerosis. Neurology 2005;65(12):1961Y1963. doi:10.1212/ 01.wnl.0000188898.02018.95.
65. Kelly VM, Nelson LM, Chakravarty EF. Obstetric outcomes in women with multiple sclerosis and epilepsy. Neurology 2009;73(22): 1831Y1836. doi:10.1212/WNL.0b013e3181c3f27d.
66. van der Kop ML, Pearce MS, Dahlgren L, et al. Neonatal and delivery outcomes in women with multiple sclerosis. Ann Neurol 2011; 70(1):41Y50. doi:10.1002/ana.22483.
67. Pasto` L, Portaccio E, Ghezzi A, et al. Epidural analgesia and cesarean delivery in multiple sclerosis post-partum relapses: the Italian cohort study. BMC Neurol 2012;12:165. doi:10.1186/1471-2377-12-165.
