Progressive Multiple Sclerosis
- Why is this article of interest?
This articles not only provides an overview of the two forms of progressive MS, Primary Progressive MS (PPMS) and secondary progressive MS (SPMS), but it also discusses the importance of early detection of progressive MS. The article also addresses the challenges health care providers face when detecting progression and other factors that may contribute to patients developing progressive MS, the importance of early detection, the need for better clinical care guidelines for people living with progressive forms of MS and when and how to modify disease treatment plans for people living with MS.
- What is progressive MS and how is it diagnosed?
Disease progression refers to the advancement of MS driven by underlying neurodegenerative processes. Myelopathy, or severe compression of the spinal cord, is a common feature found in people diagnosed with progressive MS. The pathogenesis of PPMS and SPMS is the same, and in both types, people experiencing relapses or increased MRI activity have active, progressive MS. MS progression is currently identified by using a person’s clinical history that shows the gradual worsening of disability between 6 and 12 months of onset. Additionally, vascular comorbidities may contribute to progression in MS. Unfortunately, diagnosis of either type of progressive MS, SPMS or PPMS, is often delayed.
- Are there tests to predict disease progression?
Traditionally, clinicians use imaging studies of the brain and spinal cord to measure the progression of MS. Extensive damage in certain areas of the nervous system seem to be better predictors of a higher MS-related disability burden. Better magnetic resonance imaging (MRI) tests, are being assessed to help identify progression in MS and help earlier identification of worsening disability in progressive MS. Additionally, advanced biomarkers that include measures of brain atrophy and spinal cord damage, including optical coherence tomography, a test that measures the thinning of optic nerves and serum neurofilament are promising tools for predicting progressive MS and future disability in people living with MS.
- Who is at risk for developing progressive MS?
Many different factors determine how disability evolves over time and determines the risk of people living with RRMS evolving into progressive MS, including stage of disease, age, race and ethnicity, lifestyle choices such as smoking, and the presence of other vascular diseases (comorbidities).
Smoking is a modifiable risk factor in the progression of MS and patients should be encouraged by their caregivers and health care providers to quit. An increasing number of studies in both the US and Latin America have identified a greater proportion of progressive forms and poorer disease outcomes for people of African descent. It remains unclear if these studies reflect genetic predisposition or simply shine a spotlight on greater health disparities and inequities for people of color. It has also been observed that vascular comorbidities can increase the risk of progression of MS and likely complicate treatment of progressive MS.
These risk factors are likely to worsen the disability caused by MS. Such predictors play an important role in preparing people with MS and their caregivers for any challenges that may lie ahead and help them to cope with changes in treatment plans in treating the symptoms related to progressive MS; however, before a patient is given a diagnosis of secondary progressive MS, alternative causes for their worsening symptoms should be considered.
What does this all mean for me?
Several challenges remain in the diagnosis, detection, identifying biomarkers, implementation of treatments, and care guidelines for progressive MS. Nevertheless, several relevant biomarkers look promising and could be used to determine active from inactive progressive MS. Studies of the currently available DMTs for active SPMS and PPMS underscore that younger age at onset of disease progression, and shorter disease duration, are likely to have greater benefits. Whether therapeutic control of comorbidities delays progression is unknown. Beyond treating inflammation, additional treatments that target neuroprotection and repair are needed.
For any questions, please make sure to contact your Healthcare Provider.
Original Article
Progressive Multiple Sclerosis
Continuum
Lilyana Amezcua, MD, MS, FAAN
Abstract
PURPOSE OF REVIEW
This article provides an update on progressive forms of multiple sclerosis (MS) commonly referred to as primary progressive MS and secondary progressive MS. It discusses the importance of diagnosing and detecting progression early, the similarities between progressive forms, challenges in detecting progression, factors that could augment progression, and the importance of disease-modifying therapies in patients with evidence of active progressive MS. It also discusses the overall care of progressive MS.
RECENT FINDINGS
The pathogenesis of primary progressive MS and secondary progressive MS is overlapping, and in both presentations, patients with relapses or focal MRI activity are classified as having active, progressive MS. All currently approved disease-modifying therapies are indicated for active secondary progressive MS. The therapeutic opportunity of anti-inflammatory drugs for the treatment of progressive MS is enhanced in those who are younger and have a shorter disease duration. Vascular comorbidities may contribute to progression in MS.
SUMMARY
Several challenges remain in the diagnosis, follow-up, and treatment of progressive MS. Early identification of active progressive MS is needed to maximize treatment benefit. The advantages of optimal comorbidity management (eg, hypertension, hyperlipidemia) in delaying progression are uncertain. Clinical care guidelines for advanced, severe MS are lacking.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory condition of the central nervous system (CNS) that is characterized by demyelination and concomitant axonal and neuronal degeneration. More than 2.5 million individuals are affected by MS worldwide, and MS is now considered the leading cause of atraumatic neurologic disability in young adults.1 Approximately 85% of patients with MS present with a relapsing-remitting course of the disease (relapsing remitting MS [RRMS]), and the majority of these patients, as shown in natural history studies, advance to a progressive disease course after 15 to 20 years after disease onset (secondary progressive MS [SPMS]).2 The remaining approximately 10% to 15% of patients have a slow and gradual neurologic deterioration from the onset, termed primary progressive MS (PPMS). However, progressive and relapsing forms of MS share many clinical, imaging, and pathologic features. Progression in MS can occur with or without disease activity and with or without worsening deficits on neurologic examination. It is important to clarify the application of the term progression in these cases; it is not meant to be synonymous with increasing disability over time due to poor recovery from individual relapses. Often, the terms disability progression and disease progression are used interchangeably. However, disability progression should be reserved for the accrual of physical disability, which may include that due to poor relapse recovery. Disease progression, instead, refers to the advancement of disease driven by underlying neurodegenerative processes that can co-occur with inflammatory activity but also develop in its absence. Delay in diagnosis of progressive MS is common because of the lack of defined or reliable measures of disease progression in the clinic. Definitive clinical biomarkers at the clinician’s disposal are lacking. Nevertheless, multiple possible imaging and laboratory biomarkers are being assessed, and the use of disease-modifying treatments (DMTs) in active SPMS offers a greater opportunity to intervene earlier on. The classification of progressive MS by Lublin and colleagues3 defines active as the presence of either a clinical relapse or MRI activity over a specified period of time (FIGURE5-1).
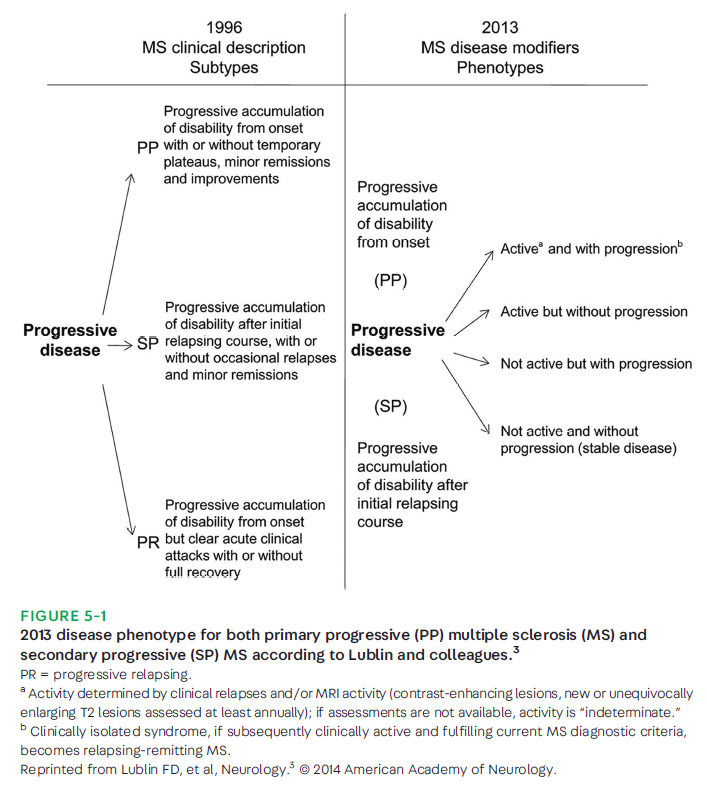
MRI activity is defined as contrast-enhancing lesions, as well as new or enlarging T2 lesions. In this article, progressive MS, challenges in detecting it, potential modifiable factors, and its treatment are reviewed.
PATHOGENESIS OF PROGRESSIVE MULTIPLE SCLEROSIS
Inflammation, which gradually subsides with age and longer disease duration, is the predominant neuropathologic feature of RRMS, whereas neurodegeneration predominates in progressive MS.3,4 Importantly, evidence now supports that both the neuroinflammatory and neurodegenerative processes can co-occur, and they often do, in an individual patient. The underlying mechanisms of primary and secondary progressive forms of MS are not completely understood but are thought to involve similar pathways, which could have therapeutic implications. The proposed mechanisms are the result of neurodegenerative processes driven by dysfunction of the innate immune system and B cells.5 In addition, oxidative stress, microglial activation, increased metabolic demand of demyelinating axons due to injury,6,7 remyelination failure, iron accumulation, and mitochondrial damage with resultant virtual hypoxia are reported, among other processes, but are beyond the scope of this article.8-10 Cortical involvement, with underlying gray matter pathology, likely contributes to disability progression in MS.11 Studies show cortical microglial activation, neuritic transection, and apoptosis without the typical perivascular lymphocytic inflammation that is characteristic of MS white matter pathology.12 The presence of subpial lesions with meningeal infiltrates containing B and T lymphocytes, plasma cells, andmacrophages is consistent with lymphoid follicles and is thought to also drive progression in MS.13 Although meningeal inflammation correlates with the severity of cortical demyelination and the appearance of these lymphoid follicles is similar to B-cell follicles, it is unclear whether these structures actively contribute to the pathology of progressive MS.13 In addition, it is hypothesized in progressive MS that inflammation that drives tissue injury has become compartmentalized behind an intact blood-brain barrier. For more information on pathophysiology, refer to the review by Faissner and colleagues13 and the article “Epidemiology and Pathophysiology of Multiple Sclerosis” by Melanie Ward, MD, and Myla D. Goldman, MD, MSc, FAAN,14 in this issue of Continuum.
CLINICAL FEATURES OF PROGRESSIVE MULTIPLE SCLEROSIS
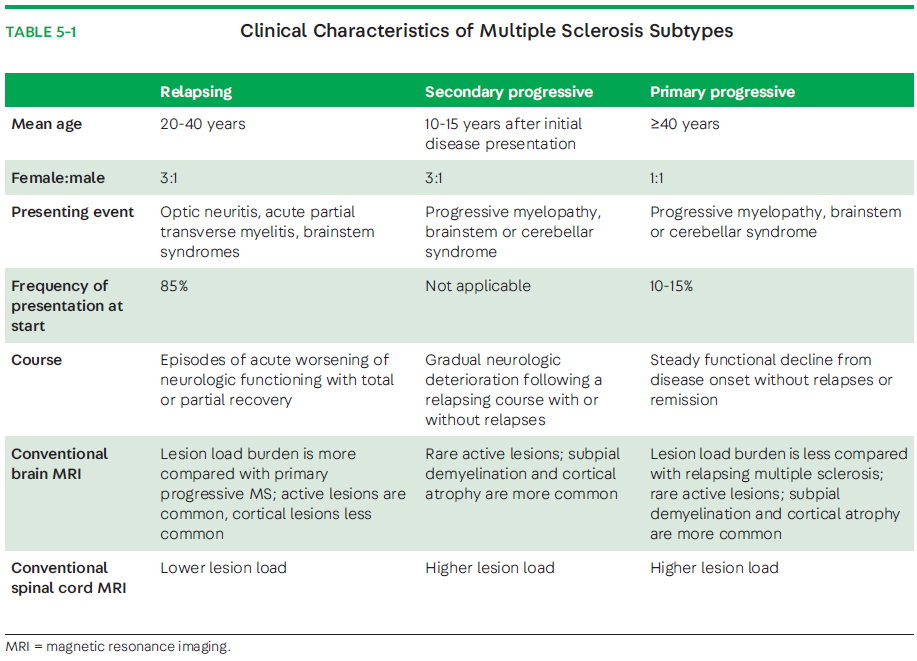
Certain clinical differences exist between the onset of RRMS and PPMS. For example, the female-to-male ratio is much closer to 1:1 in progressive-onset MS, and the mean age at onset is older (about 10 years later; 40 years old and older).3,16 This is in contrast to RRMS, where a 3:1 female-to-male predominance of MS is seen and the age of onset is between 20 and 40 years of age. In progressive MS, a progressive myelopathy is common and characterized by an insidious and worsening spastic paraparesis, usually without a discernable sensory level (80% to 85% of cases). In some cases, PPMS may present with a progressive cerebellar ataxia (10% to 15%) or other brainstem or visual symptoms (2% to 4%).17 Also, approximately 5% of adults with PPMS have symptom onset at the age of 60 years or older.18 Older individuals with MS are reportedly more likely to present with a progressive course (32%with PPMS and 23%with SPMS).18 This is in contrast to the typical RRMS presentation of optic neuritis (approximately 25%), brainstem events, and partial spinal cord syndromes that can be predominantly sensory, with or without sphincter dysfunction. Key clinical differences between the relapsing and progressive phenotypes are presented in TABLE 5-1. Progression in MS can be variable throughout the disease course, with superimposed relapses or MRI activity (ie, new or enhancing lesions) or both, as well as periods of relative disease stability. Increasing clinical, imaging, and genetic data suggest that PPMS is more similar than not to SPMS, and any pathologic differences from SPMS are relative rather than absolute. For example, a lower incidence in the global focal white matter lesion load and the presence of gadolinium-enhancing lesions is said to be found in PPMS. Nevertheless, natural history studies have shown that disability progresses at a similar rate in patients with PPMS and SPMS with or without relapses.19-21
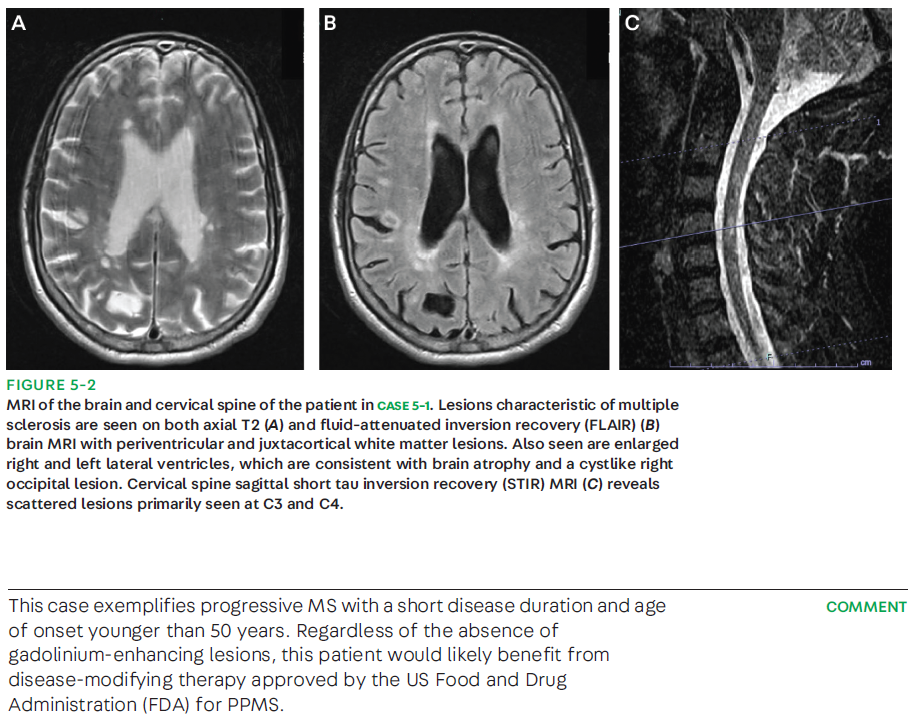
DIAGNOSING PROGRESSIVE MULTIPLE SCLEROSIS
The diagnosis of progressive MS, either SPMS or PPMS, is usually delayed. In most RRMS cases, MS progression is identified retrospectively by using clinical history that supports a gradual worsening of disability between 6 and 12 months.3,22,23 Observational studies, however, have reported a period of uncertainty of 2 to 3 years before an SPMS diagnosis is made.24,25 In 2021, a cross-sectional survey reported that neurologists who, through clinical history, observed particular changes to ambulation were willing to consider making a diagnosis of SPMS over a short patient history period (3 to 6 months).26 In contrast, the diagnosis of PPMS is much more standardized, and specific criteria exist to guide the neurologist in making the diagnosis (CASE 5-1). The 2017 McDonald criteria for PPMS require (1) 1 year of disability progression (retrospectively or prospectively determined) independent of clinical relapse, plus two of the following criteria: (1) one or more T2-hyperintense lesions on brain MRI characteristic of MS in one or more of the following brain regions: periventricular, cortical, juxtacortical, or infratentorial; (2) at least two or more T2-hyperintense lesions in the spinal cord; or (3) the presence of CSF-specific oligoclonal bands.23 The current updated criteria eliminated the distinction between symptomatic and asymptomatic lesions and emphasize the role of CSF-specific oligoclonal bands in fulfilling dissemination in time in the diagnosis of PPMS.23
When considering a diagnosis of PPMS or SPMS, it is important to exclude other causes of chronic myelopathies that can present with gradual worsening similar to progressive MS. This includes metabolic disorders such as subacute combined degeneration associated with vitamin B12 or copper deficiency, as well as adrenoleukodystrophy and mitochondrial inherited disorders. Infectious causes include neurosyphilis, progressive multifocal leukoencephalopathy (especially in patients on DMT for RRMS and who are progressing), and human T-cell lymphotropic virus type 1 (HTLV-I)-associated myelitis. A particular consideration of progressive multifocal leukoencephalopathy should be made in individuals with MS who progress while on natalizumab and in patients receiving long-term immunosuppression.27 A slowly progressive spastic paraparesis associated with human immunodeficiency virus (HIV) may also mimic the clinical course of PPMS. Inflammatory causes such as neurosarcoidosis and other systemic inflammatory conditions should also be excluded.
DETECTING PROGRESSIVE MULTIPLE SCLEROSIS
Progression in PPMS and SPMS is not uniform over time and can include periods of relative stability or slow insidious change that is difficult to measure by the patient or physician. Currently, multiple efforts are being made to identify and validate objective measures of progression, which are critically needed. At this time, no specific guidelines or satisfactory measurements of MS progression are available for use in the clinic.28,29 For years, the Expanded Disability Status Scale (EDSS) score has been the main measure of clinical MS-related disability in clinical trials.
30 Originally implemented as a research tool, the EDSS is relatively insensitive to common problems in MS outside of pyramidal dysfunction (ie, cognitive progression). The addition of more quantitative measures such as adding functional scores to the EDSS evaluation and the integration of Multiple Sclerosis Functional Composite (MSFC),31 which is a three-part, standardized, quantitative assessment instrument that originally integrated the timed 25-foot walk for leg function and ambulation, 9-hole peg test for armand hand function, and Paced Auditory Serial Addition Test (PASAT-3) for cognitive function, increased the ability to detect a reliable change over time. The Paced Auditory Serial Addition Test was replaced eventually by the Symbol Digit Modalities Test, and low-contrast assessment of visual acuity was added. In the setting of an acute relapse, disability may be reversible with resolution of focal inflammation. As such, it is important in patients with “active” progressive MS that any measured increase in disability is sustained and confirmed at 3 to 6 months.22,25 Recently, the addition of a minimum worsening of more than 20% on a timed 25-foot walk32 and 9-hole peg test33 (measure of upper extremity function) and changed EDSS at 24 weeks further delineated the separation between SPMS and nonprogression.34
Imaging
Several imaging biomarkers are under consideration to better detect andmonitor progression of MS in the clinic.35 It can be challenging to distinguish a clinical relapse from clinical progression in the clinic; currently, conventional MRI with corresponding new T2 and/or gadolinium-enhancing lesions is viewed as the best surrogate marker of clinical relapse. However, a weak correlation exists between MRI lesion load and clinical disability, which is likely related to the inability to fully measure (ie, using EDSS) and capture heterogeneity of the underlying MS pathology.36 Thus, the observation that primary progressive MS tends to have, on average, fewer T2/fluid-attenuated inversion recovery (FLAIR) lesions does not necessarily correlate to less disability. In part, this is likely because of the demonstrated findings of a shift from active to inactive plaques along with progressive atrophy of gray and white matter that is evident on MRI in primary progressive MS.37 In addition, diffuse abnormal white and gray matter of the brain and spinal cord has been reported to be more prominent in patients with progressive MS and is thought to reflect ongoing neurodegeneration and inflammation.38 Spinal cord lesions are also reported to be more common in progressive MS and are strongly associated with disability in progressiveMS.39 As with brain MRI, the ability to detect clinically relevant microstructural changes in the spinal cord with conventional MRI is needed and is being developed.39
Changes in T2-hyperintense lesion load within the first few years after disease onset are predictive of long-term disability worsening.40 Evaluation of cortical lesion burden (greater than or equal to 7) and its accumulation over time (4 years after disease onset) has been reported to predict conversion to SPMS and have clinical relevance.41 Cortical involvement, with underlying gray matter pathology, appears to be a major contributing factor to the disability seen in progressive MS.11 Nevertheless, many of these measures are still only accessible through research or in clinical trials, with heterogeneity in study methodology, which is not exclusive to differences in the strength of the MRI magnet. Hence, more advanced MRI techniques are being analyzed with the intention to better assess axonal loss by measuring gray matter atrophy, thalamic volume, spinal cord atrophy, hippocampal volume, gray matter fraction, cortical lesion quantification, and sodium imaging, among others.29,42 Thus, developing tools to readily assess these changes have the potential to be clinically relevant and feasible to predict progressive MS (TABLE 5-2).35
Optical coherence tomography (OCT) is a noninvasive tool that allows the measurements of retinal nerve fiber layer thickness, ganglion cell/inner plexiform layer thickness, and macular volume. Loss of retinal nerve fiber layer thickness and ganglion cell/inner plexiform layer thickness correlates with clinical and paraclinical parameters such as visual function, disability, and MRI in MS.43 Some studies indicate that OCT parameters may be able to predict disability progression in MS. OCT is also being considered as an outcome measure of axonal loss in several phase 2 proof-of-concept clinical trials of progressive MS.44
Other imaging and nonimaging techniques have been used in remyelination trials as biomarkers of myelin repair and include magnetization transfer ratio, diffusion-weighted imaging, myelin water imaging, and visual evoked potentials.45
Laboratory
The association between CSF oligoclonal bands and MS has been known for decades, and the presence of intrathecal oligoclonal bands is a widely used diagnostic biomarker for MS.46,47 Numerous studies have shown that, after controlling for demographic, clinical, and MRI variables, CSF oligoclonal bands are an independent predictor of a second clinical attack in an adult with clinically isolated syndrome.38-46 Patients with MS with oligoclonal bands have also been reported to have a more aggressive disease course than patients with MS without oligoclonal bands.48 However, evidence generally does not support the utility of CSF oligoclonal bands as a marker of progression from RRMS to SPMS, but oligoclonal bands are an important biomarker in the 2017 McDonald diagnostic criteria23 for PPMS and help avoid a false-positive MS diagnosis.
Recently, serum neurofilament light chain has been highlighted as a plausible marker of neurodegeneration that can be measured accurately, sensitively, and reproducibly.49-51 Findings from relapsing and progressive cohorts concur and indicate that serum neurofilament light chain concentrations correlate with CSF neurofilament light chain levels44 and imaging and disability measures predict the future course of the disease and can predict response to treatment. However, prior to its utility in progressive MS, standardization for sample processing and analysis needs to be established and the possibility that serum neurofilament light chain measurements are affected by aging and other comorbidities must be better understood.51
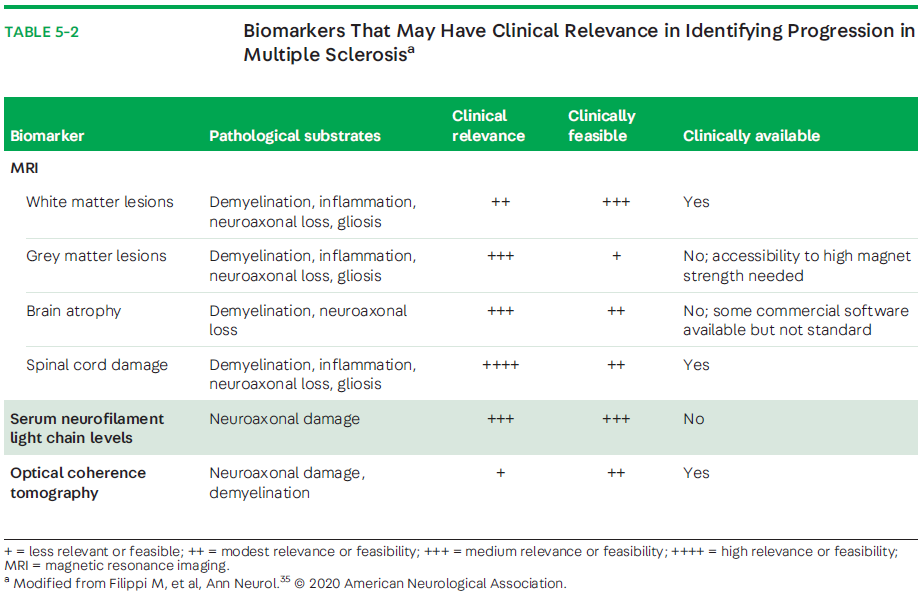
Other Clinical Measures Clinical predictors and determinants of progressive MS have been primarily quantitative, based on single-center or large-scale observational cohort studies.52,53 Nevertheless, patient-reported outcomes and real-world experience are starting to be incorporated into algorithms for evaluating the transition to progressive MS, which may enrich the physician-patient interaction in evaluating current disease state and level of progression.54 Patient age, mobility, and self-care were identified as the strongest patient-reported predictors of progression to SPMS.54 Recently, other measures of performance, such as wearable digital devices (eg, a health tracker than can be worn on the wrist) are being incorporated as additional determinations of worsening in contemporary studies.55 Limitations to detection include its retrospective nature and what definition is used to define progressive MS. MSBase, an international registry of patients with multiple sclerosis, evaluated various definitions of SPMS. The best performance definition included a three strata progression magnitude in the absence of a relapse, confirmed after 3 months within the leading Functional System and required an EDSS step 4 or greater and pyramidal score of 2 or greater.25 A current unmet need for patients and clinicians is the accurate identification of patients who have transitioned to SPMS and a validated tool to measure progression among those who are determined to have progressive MS, either PPMS or SPMS.
NONGENETIC FACTORS THAT INCREASE VULNERABILITY TO PROGRESSION IN MULTIPLE SCLEROSIS
Studies on the clinical presentation and course of MS have indicated that certain initial demographics and clinical factors are predictive of disease progression.
Race/Ethnicity
An increasing number of studies have identified African ancestry as a risk factor, not only for susceptibility to MS but also for disease progression. A greater proportion of progressive forms and poorer disease outcomes is consistently reported with African ancestry in the United States and elsewhere.56-62 Black patients with MS have been reported to have a faster transition from RRMS to SPMS.63 In Latin America, a worse prognosis for those with an African background who have primary progressive MS is also reported.59 A recent analysis of a northern California managed care network found Black patients, compared with other groups, more often had primary progressive MS (10.0% versus 0.0%to 4.0%) or progressive relapsing MS (6.0% versus 0.0%to 2.0%).61 In addition, greater structural changes in distinct anatomical regions of the brain, the visual system as noted by retinal nerve fiber layer measurements, and spinal cord (measurements of the medulla and upper cervical regions) are suggestive of greater neurodegeneration in Black individuals compared with White individuals.62,64,65
Whether these observations reflect greater encounters with health disparities and inequities or genetic predispositions is not clear.
Age
Aging is the most important risk factor for the development of neurodegenerative disease, and age-dependent degenerative processes likely contribute to MS progression in aging patients withMS.66,67 Chronologic age has been consistently associated with increased rates of MS disability accumulation and the risk of progressive MS.67 These processes are superimposed on natural brain aging processes,whichmakes it difficult to disentangle age-related changes from those due to the pathophysiology of progressive MS. Nevertheless, efforts are ongoing to determine how much normal aging contributes to brain atrophy, which may increase with age, and to the rate of MS-specific brain atrophy areas (ie, the thalamus), which may also increase with age.68Whether older age at disease onset is associated with a shorter time to disease progression has been inconclusive.69,70 Interestingly, the average age of onset of both PPMS and SPMS overlaps (around the age of 45 years), and subsequent disability progresses at a similar rate independent of age of onset and previous clinical course. A recent biomarker of aging, telomere length (using leukocyte telomere length), was found to be associated with higher levels of disability and brain atrophy in people living with MS.71
Smoking
Smoking is a modifiable risk factor in MS, and its association with disease outcomes has mostly been researched in RRMS.72 Within the progressive MS populations, the relationship between smoking and disease progression has been less clear. Some studies report that smoking is associated with a more severe disease course and faster disability progression, whereas others have not found such an association.73-75 Importantly, modification of disability following smoking cessation has been reported, supporting that it is never “too late” for a patient withMS to quit smoking.74 In 2021, the risk of conversion into secondary progressive disease was shown to increase among people who smoke when self-reported smoking history was used to define those who smoke.76 Collectively, the data support that, for an individual patient with MS, smoking may contribute to inflammatory disease activity and disease progression, as well as provide an opportunity to improve disability with cessation. All patients with MS should be encouraged and provided resources to stop smoking.
Comorbidities
The recognition is growing that comorbidities may influence disease progression in MS.77 Vascular comorbidity, whether present at symptom onset, diagnosis, or later in the disease course, has been associated with a substantially increased risk of disability progression in MS.77-82
It is possible that vascular comorbidity augments neurodegeneration by adding greater accumulation of white matter lesions and/or a diffuse hypoperfusion with consequent chronic hypoxia.82 Having type 2 diabetes, hypertension, dyslipidemia, or peripheral vascular disease has been reported to be associated with greater disability progression.78 Each cardiovascular comorbidity at disease onset increased the risk of walking disability by 50%, and patients with at least one comorbidity at diagnosis required unilateral assistance to walk a median of 6 years earlier than those without a comorbidity at diagnosis.77 A more granular look into vascular comorbidities suggests that arterial hypertension in patients with MS is associated with an increased risk of reaching higher levels of disability.79 Recently, patients with MS with systolic blood pressure variability and greater blood pressure severity (stage II as staged by the American Heart Association) were found to have greater risk of MS-related disability.80,81 More research is needed to determine the pathways in which having MS and vascular comorbidity interact. Beyond the current work focused on vascular comorbidities, additional studies around the impact of other comorbidities (eg, psychiatric comorbidities) and their impact on MS progression are needed.
PRACTICAL APPROACH TO PROGRESSIVE MULTIPLE SCLEROSIS CARE AND TREATMENT
The treatment of both PPMS and SPMS is challenging because of the heterogeneity of mechanisms involved in the pathophysiology of progression. Currently available evidence supports that CNS immune activation occurs in the early course of progressive MS with increased levels of biomarkers of demyelination and neuronal and axonal damage in CSF.1 As of 2019, all DMTs indicated for RRMS have active SPMS added to their label based on the assumption that progressive disease, still to a lesser degree, has inflammatory activity concurrent with the underlying neurodegeneration (ie, brain atrophy) despite limited effects noted in the clinical trials. Active SPMS refers to the presence of clinical relapses or MRI activity.3 No DMTs are FDA approved to treat nonactive SPMS. For more detailed information on DMTs, refer to the article “Treatment of Multiple Sclerosis” by Anne Cross, MD, FAAN, and Claire Riley, MD,83 in this issue of Continuum.
Disease-Modifying Therapies Found Effective in Studies of Progressive Multiple Sclerosis
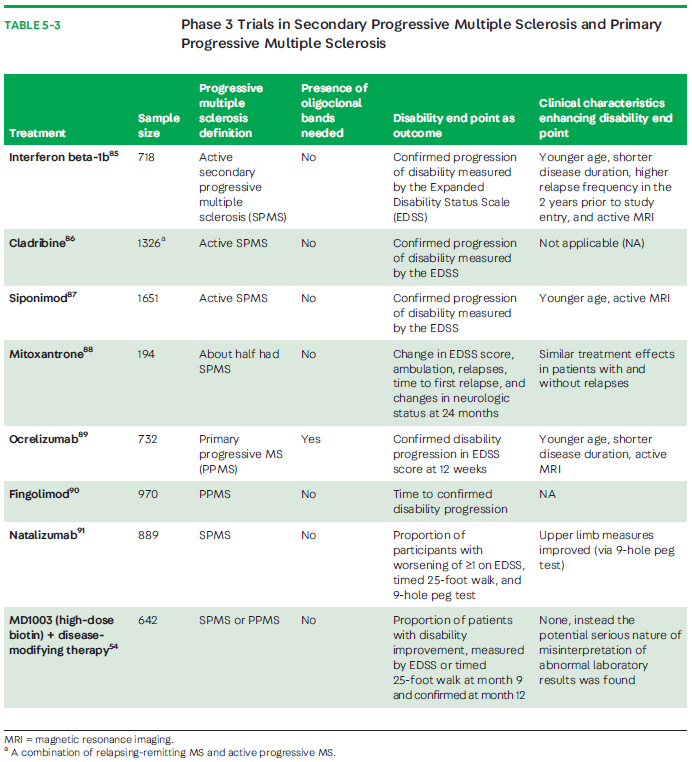
Despite all current available DMTs having the designation to treat active SPMS, only a few have been actively studied for progressive forms of MS and showed efficacy. Interferon beta medications are indicated, for example, to treat RRMS and SPMS if relapses are present. Although multiple studies, including one in the United States, were conducted to evaluate interferons, it was only a European study on SPMS that found a significant slowing in disease progression that was attributed to differences in patient characteristics (ie, more active disease) (TABLE 5-3).82,84-91 Thus, interferon beta-1b has the designation for relapsing SPMS in Europe.Mitoxantrone is a cytotoxic DNA intercalating agent that has been used as an effective DMT in MS and has been approved since the year 2000. The MIMS (Mitoxantrone in Progressive Multiple Sclerosis) trial (a double-blind, multicenter, phase 3 trial) and other clinical studies have shown a reduction in progression of disability and relapses in patients with SPMS.88 Mitoxantrone’s adverse effects, which include cardiotoxicity, bone marrow suppression, and other life-threatening hematologic malignancies, have limited its use in recent years. In 2020, a study of 10-year outcomes indicated a more favorable efficacy profile in those with RRMS compared with patients with a rapid progression.92
Siponimod is a selective modulator of sphingosine-1-phosphate receptor subtypes 1 and 5 that is approved for the treatment of active SPMS.87 It is thought to readily cross the blood-brain barrier and have direct actions in the CNS that limit inflammation and promote remyelination. Patients treated with siponimod in the EXPAND (Exploring the efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis) study experienced a significant reduction in confirmed disability progression at 3 and 6 months, especially in those with active SPMS compared with those given placebo, and less brain volume loss, which led to its approval.87 A recent post hoc analysis assessed cognitive processing speed and found siponimod to have a clinically significant benefit observed in Symbol Digits Modalities Test scores.93 In the United States, all second-generation sphingosine-1-receptor blockers (siponimod, ozanimod, ponesimod) are approved across the MS spectrum from clinically isolated syndrome to SPMS. Cladribine is a purine analogue used in the treatment of hairy cell leukemia and approved also for active SPMS.86 The CLARITY (Cladribine Tablets Treating Multiple Sclerosis Orally) study determined that cladribine significantly reduced relapse rates, risk of progression of disability, and MRI-measured disease activity in patients with RRMS and relapsing SPMS.86 However, it was not exclusively studied in SPMS.
To date, ocrelizumab is the first and only FDA-approved DMT for patients with PPMS.89 The characteristics of patients treated in ORATORIO (A Study of Ocrelizumab in ParticipantsWith Primary Progressive Multiple Sclerosis) indicate that ocrelizumab exerts an anti-inflammatory effect most pronounced in those who were younger than 55 years of age and with evidence of higher disease activity (27.5% of those treated with ocrelizumab had gadolinium-enhancing lesions onMRI).89,94 This idea is supported in the rituximab clinical trial in PPMS that also showed greater benefit in those younger than 50 years of age and with gadolinium-enhancing lesions on MRI.95 Thus, a stronger consideration should be given to treat those younger and in early stages of PPMS.
Results of studies that were designed to specifically assess efficacy of glatiramer acetate, fingolimod, and natalizumab in either PPMS or nonactive SPMS have been dissapointing.90,91,96,97 Part of their failure to show benefit has been attributed to trial design, trial duration, inclusion criteria, and selection of appropriate clinical and imaging outcome measures.29
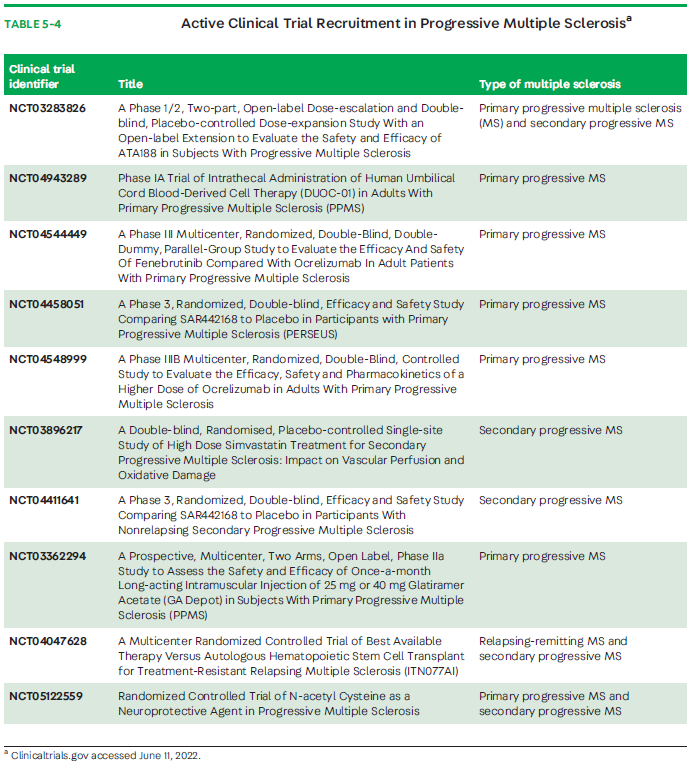
Trials focusing on mechanistic aspects of neurodegeneration in progressive MS could be more promising. Unfortunately, the recent results from a study of MD1003 (high-dose biotin), thought to enhance neuronal and oligodendrocyte energetics, resulting in improved cell function, repair, or survival, did not show benefit.55 Adding MD1003 to existing DMT compared with placebo in patients with nonactive progressive MS (PPMS or SPMS) provided no improvement of MS-related disability or prevention of worsening disability measured by the EDSS.55 Efforts to evaluate therapeutics that more specifically target inflammation compartmentalized to the CNS or neurodegeneration are at various stages of development. Of these, ibudilast, a small molecule able to cross the blood-brain barrier, is a promising investigational therapeutic; compared with placebo, it slowed the progression of brain atrophy in patients with progressiveMS (PPMS or SPMS) in a phase 2 study.98 Additional smallmolecules being investigated with the capacity to cross the blood-brain barrier include the Bruton tyrosine kinase (BTK) inhibitors. Current clinical trials actively recruiting patients with SPMS and PPMS are shown in TABLE 5-4.
Approach to Nonactive Stable Progressive Multiple Sclerosis
Challenges and uncertainty still exist in treating disease progression in patients without evidence of clinical relapses or MRI activity. No formal guidelines have been established surrounding when and how to stop DMTs in a patient with stable nonactive progressive MS. However, data support that safely stopping the use of DMTs for people with nonactive progressive MS is possible. Most studies indicate that older age, longer periods of stable disease on DMT with absent relapses or MRI activity, and an EDSS score less than 6 are associated with a low risk of disease recrudescence and worsening when stopping DMT use. In contrast, younger age (younger than 45 years) and a higher number of inflammatory lesions on MRI are predictive of resumption of disease activity.99,100 Discontinuation of DiseaseModifying Therapies inMultiple Sclerosis (not recruiting) is a prospective interventional randomized study that includes patients with MS who are 55 years old or older, including both progressive forms of MS, and no evidence of activity.101 In this study, nonactive is defined as no evidence of recent new inflammatory diseasewith no relapse for at least 5 years and no newMRI lesion for at least 3 years. The primary outcome is the number of patients developing new disease activity with discontinuation of DMT. The results are expected in the next year and will provide important guidance to patients and clinicians who are now caring for an aging population in the therapeutic era of MS.
The short- and long-term potential toxicity and cost of DMTs need to be carefully considered in aging patientswho haveMS and in thosewith progressive MS. Similarly, the risk of stopping DMTs in those with significant disability and active disease would be a significant risk. If discontinuation of DMT is decided, continuedmonitoring for breakthrough disease is highly recommended. It is also important to recognize the risk of inflammatory activity with the stopping of certain agents (eg, natalizumab and fingolimod), known as rebound, in those who would otherwise be candidates for discontinuation.
MULTIDISCIPLINARY CARE AND CONTEMPORARY CONSIDERATIONS IN THE CARE OF PROGRESSIVE MULTIPLE SCLEROSIS
Patients with progressive MS have complex physical and psychosocial needs, which can make general care challenging. If identification of active progressive MS is present, switching to a higher-efficacy medication in those already on a DMT is strongly recommended (CASE 5-2). Regular patient visits and familiarity with rehabilitative strategies and symptom management are also needed.102
Cognitive decline; falls; bladder, bowel, and sexual impairments; fatigue; and mood changes are common symptoms that worsen over time. This includes worsening cognitive fatigue and depression, which are symptoms that have been reported to negatively affect quality of life for patients with MS.1,3 Routine evaluations should include screening for these symptoms and evaluating and treating for new ones, such as pseudobulbar affect (pathologic laughing and crying, approximately 10% of MS cases), which mainly occurs in progressive MS. Other conditions that can mimic or augment progression include musculoskeletal conditions that have developed as a consequence of MS ambulatory disability (eg, meniscal tears, knee and hip injuries) or independent of MS (ie, spinal stenosis and other medical comorbidities such as vascular disease). Before a patient is given a diagnosis of SPMS, alternative causes for their worsening symptoms should be sought. This includes deconditioning as a result of lockdown measures during the COVID-19 pandemic and the related postponements and delays in care, including DMT changes.103,104 Many patients with progressive disease have become more restricted to home during the COVID-19 pandemic and lost the opportunity for physical therapy and/or gym participation, which has led to generalized worsening that can be misclassified as progression. It is important to consider these possibilities because they can represent sources of “reversible” progression for patients.
A multidisciplinary approach to care is recommended. This includes disciplines such as physical therapy, occupational therapy, urology, pain management, primary care, social services, counseling, and neuro-ophthalmologic evaluations. The high prevalence of motor disorders and gait disabilities, the negative impact on personal activities and quality of life, and the limited effects of specific medications make gait rehabilitation a crucial part of the management. Encouragement to follow a healthy diet, exercise regularly and stretch, and maintain sleep hygiene is also recommended. Rehabilitation support should also include caregiver support services, tending to the emotional needs of both the patient and caregiver, and supportive services to assist with the physical and cognitive deficits being experienced by the patient with progressive MS.105 For more information on symptom management, refer to the article “Approach to Symptom Management of Multiple Sclerosis With a Focus on Wellness” by Rebecca Spain, MD, MSPH, FAAN,106 in this issue of Continuum.
Treatment in severe cases of progressive MS with disability levels where the patient is restricted to a bed (an EDSS score of 8 or more) entails considering palliative care. Palliative care is an interdisciplinary approach that improves the quality of life of patients and their families facing the problems associated with incurable diseases or life-threatening illnesses, through the prevention and relief of symptoms by means of early identification and impeccable assessment and treatment of physical and psychosocial concerns.107 Those with severe progressiveMS could benefit frompalliative care approacheswhen the accrual of physical and cognitive disability has been significant. An exponential increase in the use of palliative care for hospitalized patients with MS was reported over a 10-year period (0.2% to 6.1%from2005 to 2014),mostly in those at higher risk of in-hospital death.108 Palliative care guidelines are being developed to help respond to the complex and varying physical and psychosocial needs of patients with severe progressive MS.109,110 It is important to begin conversations early with patients and their family members about their wishes and plans for when their disability reaches a threshold that requires full-time care and loss of independent activities of daily living.
CONCLUSION
Several challenges remain in the diagnosis, detection, biomarkers, implementation of treatments, and care guidelines for progressive MS. Nevertheless, several relevant biomarkers look promising and could be used to distinguish active from inactive progressive MS in the clinic. Studies of the currently available DMTs for active SPMS and PPMS underscore that younger age at onset and shorter disease duration are likely to have greater benefit. Whether therapeutic control of comorbidities delays progression is unknown. Beyond inflammation, additional treatments that target neuroprotection and repair are needed. Multidisciplinary care is crucial in caring for patients with progressive MS, including the need to better understand where palliative care fits into the care algorithm for severe progressive MS.
REFERENCES
1 Mackenzie I, Morant S, Bloomfield G, et al. Changing face of multiple sclerosis in the United Kingdom 1990–2010. An incidence and prevalence study. J Neurol Neurosurg Psychiatry 2013;84(11):e2. doi:10.1136/jnnp-2013-305450
2 Sellebjerg F, Bornsen L, Ammitzboll C, et al. Defining active progressive multiple sclerosis. Mult Scler 2017;23(13):1727-1735. doi: 10.1177/1352458517726592
3 Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560
4 Lassmann H, Horssen J, Mahad D, et al. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 2012;8(11):647-656. doi:10.1038/nrneurol.2012.168
5 Baecher-Allan C, Kaskow BJ, Weiner HL, et al. Multiple sclerosis: mechanisms and immunotherapy. Neuron 2018;97(4):742-768. doi: 10.1016/j.neuron.2018.01.021
6 Mahad DH, Trapp BD, Lassmann H, et al. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015;14(2):183-193. doi: 10.1016/S1474-4422(14)70256-X
7 Trapp BD, Peterson J, RansohoffRM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338(5):278-285. doi:10.1056/ NEJM199801293380502
8 Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol 2009;8(3):280-291. doi: 10.1016/S1474-4422(09)70043-2
9 Heidker RM, Emerson MR, LeVine SM, et al. Metabolic pathways as possible therapeutic targets for progressive multiple sclerosis. Neural Regen Res 2017;12(8):1262-1267. doi:10.4103/1673- 5374.213542
10 Hametner S, Wimmer I, Haider L, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013;74(6):848-861. doi:10.1002/ ana.23974
11 Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012; 135(Pt 10):2952-2961. doi:10.1093/brain/aws246
12 Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50(3):389-400. doi:10.1002/ana.1123
13 Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011;134(Pt 9):2755-2771. doi:10.1093/brain/ awr182
14 Faissner S, Plemel JR, Gold R, Wee Yong V. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov 2019;18(12):905-922. doi: 10.1038/s41573-019-0035-2
15 Ward M, Goldman MD. Epidemiology and phathophysiology of multiple sclerosis. Continuum (Minneap Minn) 2022: 28(4, Multiple Sclerosis and Related Disorders): 988-1005.
16 Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46(4):907-911. doi:10.1212/ wnl.46.4.907
17 Rice CM, Cottrell D, Wilkins A, Scolding NJ. Primary progressive multiple sclerosis: progress and challenges. J Neurol Neurosurg Psychiatry 2013;84(10):1100-1106. doi:10.1136/jnnp-2012- 304140
18 Bermel RA, Rae-Grant AD, Fox RJ, et al. Diagnosing multiple sclerosis at a later age: more than just progressive myelopathy. Mult Scler 2010;16(11):1335-1340. doi: 10.1177/1352458510377334
19 Ebers GC. Natural history of primary progressive multiple sclerosis. Mult Scler 2004;10(Suppl 1): S8-S13. doi:10.1191/1352458504ms1025oa
20 Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129(Pt 3):606-616. doi:10.1093/brain/awl007
21 Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010;133(Pt 7):1900-1913. doi: 10.1093/brain/awq076
22 Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015;138(Pt 11):3287-3298. doi:10.1093/brain/ awv258
23 Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17(2): 162-173. doi:10.1016/S1474-4422(17)30470-2
24 Katz Sand I, Krieger S, Farrell C, Miller A. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler 2014;20(12):1654-1657. doi: 10.1177/1352458514521517
25 Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain 2016;139(Pt 9):2395-2405. doi: 10.1093/brain/aww173
26 Alvarez E, Nair KV, Gorritz M, et al. Identification and diagnosis of secondary progressive multiple sclerosis during the clinical encounter: results from a physician survey. Mult Scler Relat Disord 2021;50:102858. doi:10.1016/j.msard.2021.102858
27 Pitarokoili K, Gold R. Multiple sclerosis: progressive multifocal leukoencephalopathy risk stratification. Nat Rev Neurol 2017;13(12):710-712. doi:10.1038/nrneurol.2017.161
28 Tavazzi E, Zivadinov R, Dwyer MG, et al. MRI biomarkers of disease progression and conversion to secondary-progressive multiple sclerosis. Expert Rev Neurother 2020;20(8): 821-834. doi:10.1080/14737175.2020.1757435
29 Ontaneda D, Fox RJ, Chataway J, et al. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol 2015;14(2):208-223. doi:10.1016/S1474-4422(14) 70264-9
30 Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444-1452. doi: 10.1212/wnl.33.11.1444
31 Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999;5(4):244-250. doi: 10.1177/135245859900500409
32 Goldman MD, Motl RW, Scagnelli J, et al. Clinically meaningful performance benchmarks in MS: timed 25-foot walk and the real world. Neurology 2013;81(21):1856-1863. doi:10.1212/01. wnl.0000436065.97642.d2
33 Orbach R, Zhao Z, Wang YC, O’Neill G, Cadavid D. Comparison of disease activity in SPMS and PPMS in the context of multicenter clinical trials. PLoS One 2012;7(10):e45409. doi:10.1371/journal. pone.0045409
34 Cadavid D, Cohen JA, Freedman MS, et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler 2017;23(1):94-105. doi: 10.1177/1352458516638941
35 FilippiM, Preziosa P, Langdon D, et al. Identifying progression in multiple sclerosis: new perspectives. Ann Neurol 2020;88(3):438-452. doi:10.1002/ana.25808
36 Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002;15(3):239-245. doi:10.1097/00019052- 200206000-00003
37 Bodini B, Chard D, Altmann DR, et al. White and gray matter damage in primary progressive MS: the chicken or the egg? Neurology 2016;86(2): 170-176. doi:10.1212/WNL.0000000000002237
38 Nijeholt GJ, Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain 1998; 121(Pt 4):687-697. doi:10.1093/brain/121.4.687
39 Oh J, Saidha S, Chen M, et al. Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology 2013;80(6):540-547. doi:10.1212/ WNL.0b013e31828154c5
40 Tintore M, Rovira A, Rio J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015; 138(Pt 7):1863-1874. doi:10.1093/brain/awv105
41 Scalfari A, Romualdi C, Nicholas RS, et al. The cortical damage, early relapses, and onset of the progressive phase in multiple sclerosis. Neurology 2018;90(24):e2107-e2118. doi:10.1212/ WNL.0000000000005685
42 Mahajan KR, Ontaneda D. The role of advanced magnetic resonance imaging techniques in multiple sclerosis clinical trials. Neurotherapeutics 2017;14(4):905-923. doi: 10.1007/s13311-017-0561-8
43 Costello F, Burton JM. Retinal imaging with optical coherence tomography: a biomarker in multiple sclerosis? Eye Brain 2018;10:47-63. doi: 10.2147/EB.S139417
44 Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89(22):2230-2237. doi:10.1212/ WNL.0000000000004683
45 Oh J, Ontaneda D, Azevedo C, et al. Imaging outcome measures of neuroprotection and repair in MS: a consensus statement from NAIMS. Neurology 2019;92(11):519-533. doi: 10.1212/WNL.0000000000007099
46 Simonsen CS, FlemmenHO, Lauritzen T, et al. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult Scler J Exp Transl Clin 2020;6(1):2055217319901291. doi: 10.1177/2055217319901291
47 Hegen H, Zinganell A, Auer M, Deisenhammer F. The clinical significance of single or double bands in cerebrospinal fluid isoelectric focusing. A retrospective study and systematic review. PLoS One 2019;14(4):e0215410. doi:10.1371/journal. pone.0215410
48 Rojas JI, Tizio S, Patrucco L, Cristiano E. Oligoclonal bands in multiple sclerosis patients: worse prognosis? Neurol Res 2012;34(9):889-892. doi:10.1179/1743132812Y.0000000088
49 Siller N, Kuhle J, Muthuraman M, et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler 2019;25(5):678-686. doi: 10.1177/1352458518765666
50 Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81(6):857-870. doi:10.1002/ana.24954
51 Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 2020;95(10): 436-444. doi:10.1212/WNL.0000000000010346
52 Manouchehrinia A, Zhu F, Piani-Meier D, et al. Predicting risk of secondary progression in multiple sclerosis: a nomogram. Mult Scler 2019; 25(8):1102-1112. doi:10.1177/1352458518783667
53 Skoog B, Tedeholm H, Runmarker B, Oden A, Anderson A. Continuous prediction of secondary progression in the individual course of multiple sclerosis. Mult Scler Relat Disord 2014;3(5): 584-592. doi:10.1016/j.msard.2014.04.004
54 Tolley C, Piani-Meier D, Bentley S, et al. A novel, integrative approach for evaluating progression in multiple sclerosis: development of a scoring algorithm. JMIR Med Inform 2020;8(4):e17592. doi:10.2196/17592
55 Cree BAC, Cutter G, Wolinsky JS, et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2020;19(12):988-997. doi:10.1016/S1474-4422(20)30347-1
56 Khan O, Williams MJ, Amezcua L, et al. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract 2015;5(2): 132-142. doi:10.1212/CPJ.0000000000000112
57 Amezcua L, Rivas E, Joseph S, Zhang J, Liu L. Multiple sclerosis mortality by race/ethnicity, age, sex, and time period in the United States, 1999-2015. Neuroepidemiology 2018;50(1-2): 35-40. doi:10.1159/000484213
58 Amezcua L, McCauley JL. Race and ethnicity on MS presentation and disease course. Mult Scler 2020;26(5):561-567. doi:10.1177/1352458519887328
59 Ferreira Vasconcelos C, Santos Thuler L, Cruz dos Santos G, et al. Differences in the progression of primary progressive multiple sclerosis in Brazilians of African descent versus white Brazilian patients. Mult Scler 2010;16(5):597-603. doi:10.1177/1352458509360987
60 Sidhom Y, Maillart E, Montcel S, et al. Fast multiple sclerosis progression in North Africans: both genetics and environment matter. Neurology 2017;88(13):1218-1225. doi:10.1212/ WNL.0000000000003762
61 Romanelli RJ, Huang Q, Lacy J, et al. Multiple sclerosis in a multi-ethnic population from Northern California: a retrospective analysis, 2010-2016. BMC Neurol 2020;20(1):163. doi: 10.1186/s12883-020-01749-6 CONTINUUMJOURNAL.
62 Moog TM, McCreary M, Stanley T, et al. African Americans experience disproportionate neurodegenerative changes in the medulla and upper cervical spinal cord in early multiple sclerosis.Mult Scler Relat Disord 2020;45:102429. doi:10.1016/j.msard.2020.102429
63 Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult Scler 2003; 9(3):293-298. doi:10.1191/1352458503ms909oa
64 Caldito N, Saidha S, Sotirchos E, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain 2018;141(11):3115-3129. doi: 10.1093/brain/awy245
65 Kimbrough DJ, Sotirchos ES, Wilson JA, et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol 2015;77(2):228-236. doi:10.1002/ana.24308
66 Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019;15(10):565-581. doi:10.1038/s41582- 019-0244-7
67 Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology 2011;77(13): 1246-1252. doi:10.1212/WNL.0b013e318230a17d
68 Azevedo CJ, Cen SY, Jaberzadeh A, et al. Contribution of normal aging to brain atrophy in MS. Neurol Neuroimmunol Neuroinflamm 2019; 6(6):e616. doi:10.1212/NXI.0000000000000616
69 Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010;74(24): 2004-2015. doi:10.1212/WNL.0b013e3181e3973f
70 Newbould RD, Nicholas R, Thomas CL, et al. Age independently affects myelin integrity as detected by magnetization transfer magnetic resonance imaging in multiple sclerosis. Neuroimage Clin 2014;4:641-648. doi:10.1016/j. nicl.2014.02.004
71 Krysko KM, Henry RG, Cree BAC, et al. Telomere length is associated with disability progression in multiple sclerosis. Ann Neurol 2019;86(5):671-682. doi:10.1002/ana.25592
72 Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors andmultiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lacet Neurol 2015;14(3):263-273. doi:10.1016/S1474-4422(14)70267-4
73 Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol 2009;66(7):858-864. doi:10.1001/ archneurol.2009.122
74 Manouchehrinia A, Tench CR, Maxted J, et al. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013;136(Pt 7):2298-2304. doi:10.1093/brain/ awt139
75 Koch M, Harten A, Uyttenboogaart M, De Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology 2007;69(15):1515-1520. doi: 10.1212/01.wnl.0000277658.78381.db
76 Hedstrom AK, Olsson T, Alfredsson L, et al. Cotinine as a measure of smoking in observational studies of multiple sclerosis. Mult Scler 2021;27(8):1293-1296. doi: 10.1177/1352458520968803
77 Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;30;74(13):1041-1047. doi:10.1212/ WNL.0b013e3181d6b125
78 Tettey P, Simpson S Jr, Taylor BV,Mei IA. Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci 2014;347(1-2): 23-33. doi:10.1016/j.jns.2014.10.020
79 Dagan A, Gringouz I, Kliers I, Segal G. Disability progression in multiple sclerosis is affected by the emergence of comorbid arterial hypertension. J Clin Neurol 2016;12(3):345-350. doi:10.3988/jcn.2016.12.3.345
80 Goldman MD, Min S, Lobo JM, Sohn MW. Retrospective cohort study of the relationship between systolic blood pressure variability and multiple sclerosis disability. BMJ Open 2020; 10(2):e034335. doi:10.1136/bmjopen-2019-034355
81 Robers MV, Chan C, Vajdi B, et al. Hypertension and hypertension severity in Hispanics/Latinx with MS. Mult Scler 2021;27(12):1894-1901. doi: 10.1177/:13524585211019243
82 Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017;13(6):375-382. doi:10.1038/nrneurol.2017.33
83 Cross A, Riley C. Treatment of multiple sclerosis. Continuum (Minneap Minn) 2022; 28(4, Multiple Sclerosis and Related Disorders): 1025-1051.
84 Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: clinical results. Neurology 2001;56(11):1496-1504. doi:10.1212/ wnl.56.11.1496
85 Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet 1998;352(9139):1491-1497.
86 Giovannoni G, Comi G, Cook S, et al. A placebocontrolled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010;362(5): 416-426. doi:10.1056/NEJMoa0902533
87 Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018; 391(10127):1263-1273. doi:10.1016/S0140-6736(18) 30475-6
88 Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002;360(9350): 2018-2025. doi:10.1016/S0140-6736(02)12023-X
89 Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressivemultiple sclerosis. N Engl JMed 2017; 376(3):209-220. doi:10.1056/NEJMoa1606468
90 Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016;387(10023):1075-1084. doi:10.1016/S0140- 6736(15)01314-8
91 Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol 2018;17(5): 405-415. doi:10.1016/S1474-4422(18)30069-3
92 Foo EC, Russell M, Lily O, Ford HL. Mitoxantrone in relapsing-remitting and rapidly progressive multiple sclerosis: ten-year clinical outcomes post-treatment with mitoxantrone. Mult Scler Relat Disord 2020;44:102330. doi:10.1016/j. msard.2020.102330
93 Benedict RHB, TomicD, Cree BA, et al. Siponimod and cognition in secondary progressive multiple sclerosis: EXPAND secondary analyses. Neurology 2021;96(3):e376-e386. doi:10.1212/ WNL.0000000000011275
94 Holloman JP, Axtell RC, Monson NL, Wu GF. The role of B cells in primary progressive multiple sclerosis. Front Neurol 2021;12:680581. doi: 10.3389/fneur.2021.680581
95 Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009;66(4):460-471. doi:10.1002/ ana.21867
96 Wolinsky JS, Narayana PA, O’Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007;61(1):14-24. doi:10.1002/ana.21079
97 Rice GP, Filippi M, Comi G, et al. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. CladribineMRI Study Group. Neurology 2000;54(5):1145-1155. doi: 10.1212/wnl.54.5.1145
98 Fox RF, Coffey CS, Conwit R, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med 2018;379(9):846-855. doi:10.1056/ NEJMoa1803583
99 Kaminsky AL, Omorou AY, Soudant M, et al. Discontinuation of disease-modifying treatments formultiple sclerosis in patients aged over 50 with disease inactivity. J Neurol 2020; 267(12):3518-3527. doi:10.1007/s00415-020- 10029-9
100 Hartung HP, Meuth SG, Miller DM, Comi G. Stopping disease-modifying therapy in relapsing and progressive multiple sclerosis. Curr Opin Neurol 2021;34(4):598-603. doi: 10.1097/WCO.0000000000000960
101 ClinicalTrials.gov. Discontinuation of disease modifying therapies in multiple sclerosis (NCT03073603). Updated December 20, 2021. Accessed June 15, 2022. clinicaltrials.gov/ct2/ show/NCT03073603
102 Oh J, Alikhani K, Bruno T, et al. Diagnosis and management of secondary-progressive multiple sclerosis: time for change. Neurodegener Dis Manag 2019;9(6):301-317. doi: 10.2217/nmt-2019-0024
103 Donze C, Massot C, Kwiatkowski A, et al. CONFISEP: impact of the Covid-19 pandemic lockdown on patients with multiple sclerosis in the north of France. Rev Neurol (Paris) 2022; 178(1-2):151-155. doi:10.1016/j.neurol.2021.09.001
104 Mateen FJ, Rezaei S, Alakel N, et al. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol 2020;267(12):3467-3475. doi:10.1007/ s00415-020-10045-9Mateen
105 Clare L, Teale JC, Toms G, et al. Cognitive rehabilitation, self-management, psychotherapeutic and caregiver support interventions in progressive neurodegenerative conditions: a scoping review. NeuroRehabilitation 2018;43(4):443-471. doi: 10.3233/NRE-172353
106 Spain R. Approach to symptom management of multiple sclerosis with a focus on wellness. Continuum (Minneap Minn) 2022; 28(4, Multiple Sclerosis and Related Disorders): 1052-1082.
107 WachtermanMW, Pilver C, Smith D, et al. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med 2016;176(8):1095-1102. doi:10.1001/ jamainternmed.2016.1200
108 Lee YJ, Yoo JW, Hua L, et al. Ten-year trends of palliative care utilization associated with multiple sclerosis patients in the United States from 2005 to 2014. J Clin Neurosci 2018;58:13-19. doi:10.1016/j.jocn.2018.10.082
109 Oliver DJ, Borasio GD, Caraceni A, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol 2016;23(1):30-38. doi:10.1111/ene.12889
110 Solari A, Giordano A, Sastre-Garriga J, et al. EAN guideline on palliative care of people with severe, progressive multiple sclerosis. J Palliat Med 2020;23(11):1426-1443. doi:10.1089/ jpm.2020.0220
