What People Are Talking About: Recent Achievements in Stem Cell-Mediated Myelin Repair
Why is this important to me?
Drugs currently used to manage MS modify the immune system in some way. Although they can be effective at reducing relapses, they do not help to repair existing damage to the brain and spinal cord. No therapies are available that protect the brain from new damage or induce repair of damaged tissue, and new strategies are needed. The lack of therapies is especially problematic if you have a progressive form of MS.
What is the objective of this study?
The cells and molecules needed to repair damage in the brain caused by MS are present. Naturally occurring enzymes and proteins, however, seem to prevent these cells from functioning well, and the resulting repair is almost always incomplete and slow. Thus, new research is aimed at promoting natural repair by removing those substances that prevent cells from repairing the brain and providing new, transplanted cells that can repair damage. It is important to note that these results reflect experiments on very small numbers of individuals. They have not been approved for use in patients.
One component of the brain that is destroyed in MS is called myelin, the fat-like substance that surrounds and protects nerve fibers. Nerve fibers that have lost their myelin do not function properly and lead to the presentation of symptoms. Myelin in the brain and spinal cord is made by cells called oligodendrocytes. Surviving oligodendrocytes likely do not perform repair of myelin. Instead, new cells must be introduced into the damaged area. New oligodendrocytes can be developed from immature cells or from adult neural stem cells that are already present in brain. Cells that perform repair must be able to relocate to areas of damage and mature into the right cell type.
Several inhibitory molecules are present in the brain that block repair. One strategy is to overcome this inhibiting activity with new drugs. Currently available disease-modifying therapies can be effective if you have the
relapsing-remitting form of MS. The strategies described below are experimental and are associated with certain unanswered questions such as whether all MS lesions can be repaired with these strategies, if they are superior to current treatments, and if the cells and molecules involved can be efficiently delivered to damaged areas in the MS brain. Nevertheless, protecting the brain from new damage and repairing existing damage are very important, especially if your MS is progressive.
Drugs being tested in laboratory animals and clinical trials include:
● Fingolimod: This first oral medication approved to treat relapsing-remitting MS has beneficial effects on immune cells and also mediates oligodendrocyte survival, proliferation, and maturation, as well as myelin repair.
● GNbAC1: This antibody blocks another type of molecule that inhibits maturation of oligodendrocyte precursor cells.
● Anti-Sema4 antibody: This antibody prevents unwanted substances from entering the brain and promotes relocation of oligodendrocyte precursor cells to the site of damage.
● Statins: Statins are a class of drugs used to lower cholesterol. Some statins may also help oligodendrocyte precursor cells to survive and mature.
● Several other molecules are also being tested for similar activities.
Another strategy is cell replacement. Stem cells, which can either divide to create new stem cells or mature into a specific type of cell, may be transplanted into sites of damage and repair the damage. Transplanted cells can also help to ensure that nearby immune cells to not attack the brain or secrete molecules that help with the repair process.
● Mesenchymal stem cells, which are found in the bone marrow and other tissues, can increase fat, bone, muscle, and skin cells, secrete factors that promote maturation of oligodendrocyte precursor cells and protect nerve cells from additional damage. The effects of mesenchymal stem cells are being tested in laboratory models and clinical trials.
● Transplantation of adult-derived neural stem cells or oligodendrocyte precursor cells may restore the myelin-making cell population. The effects of these cells are being tested in laboratory models and clinical trials.
● Another possibility is induced pluripotent stem cells, which are derived from skin cells. However, technical problems and safety concerns are currently hampering the use of these cells in humans.
Currently available disease-modifying therapies can be effecive if you have the relapsing-remitting form of MS. The strategies described above are experimental and are associated with certain unanswered questions such as whether all MS lesions can be repaired with these strategies, if they are superior to current treatments, and if the cells and molecules involved can be efficiently delivered to damaged areas in the MS brain. Nevertheless, protecting the brain from new damage and repairing existing damage are very important, especially if your MS is progressive.
How did the authors study this issue?
The authors reviewed strategies being tested in animal models and clinical trials.
Original Article
Recent Achievements in Stem Cell-Mediated Myelin Repair
Current Opinion in Neurology
Janusz Joachim Jadasza , Catherine Lubetzki b,c,d,e, Bernard Zalcb,c,d, Bruno Stankoff b,c,d,e, Hans-Peter Hartunga , and Patrick Ku¨rya
INTRODUCTION
Multiple sclerosis is a chronic inflammatory demyelinating disease of the central nervous system (CNS) and is characterized by damage and loss of myelin sheaths and oligodendrocytes. As these axon-glia interactions build the structural base for accelerated nerve conduction and have furthermore been recognized to be important for axonal nutrition [1], their disturbance leads to a variety of symptoms such as visual impairment, loss of sensation and paralysis up to cognitive deficiencies. Pathophysiologically, multiple sclerosis is thought to be driven by autoimmune responses targeting mainly myelinated axons and oligodendrocytes. The underlying reasons and mechanisms are far from being understood, but vigorous neurodegenerative processes are also suspected to contribute, and certainly govern evolving permanent deficits and disability in progressive disease forms [2]. The course of multiple sclerosis varies and has traditionally been subdivided into relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS) and primary progressive multiple sclerosis (PPMS). A number of therapeutic approaches for multiple sclerosis have been identified and are currently applied mainly in the treatment of RRMS patients. These strategies include general immunomodulation/suppression, modulation of immune cell egress from lymph nodes, their penetration into brain parenchyma up to neutralization and depletion of specific immune cell types [3]. In light of these highly effective treatments currently at disposal to the neurologist, research has focused to unresolved issues such as neuroprotection and repair of demyelinated lesions. Although addressing existing damage is an ultimate therapeutic need, currently no treatments are available, and this limits management options for patients with progressive disease forms. Multiple sclerosis brain histopathological analyses [4] and studies on immune-mediated and toxin-mediated animal models of demyelination [5] revealed that in the adult CNS endogenous regeneration activities exist. Nonetheless, particularly upon inflammation, repair efficiencies are low and tend to diminish during disease progression. Therapeutic approaches should, therefore, either address such endogenous cell populations or provide the injured CNS with repair-mediating cells from outside.
KEY POINTS:
- Highly effective treatments for RRMS patients are currently available.
- Unresolved multiple sclerosis issues are neuroprotection and myelin repair, and this limits management options for patients with progressive disease forms.
- Endogenous myelin remyelination activities can be observed; however, they remain inefficient.
- Remyelination therapies either aim at supporting endogenous progenitor and/or stem cell populations in successfully generating new oligodendrocytes or rely on exogenous supply of repair-mediating stem cell types.
PROMOTION OF ENDOGENOUS REPAIR ACTIVITIES
Although the adult CNS is generally regarded as a regeneration incompetent organ, few repair activities can be observed, most notably the replacement of oligodendrocytes and myelin sheaths following demyelination or injury. Mature oligodendrocytes are highly vulnerable and in general degenerate due to primary insult or secondarily as a consequence of oxidative and excitotoxic stress. As these mature cells are unlikely to contribute successfully to myelin repair [6& ], immature cells such as resident oligodendroglial precursor cells (OPCs) [7] or adult neural stem cells (aNSCs) [8,9&&,10,11] jump in, become activated and are recruited in order to replace lost myelin sheaths and to restore axonal functionality. This regenerative potential is remarkable with the downside that myelin repair is also confronted with a number of limitations and in many instances remains inefficient or even fails – much alike the well-known impairment of axonal regeneration in the adult CNS [12].
generation in the adult CNS [12]. For successful tissue restoration, precursor and stem cells need to be attracted to lesions where differentiation, interactions with axons as well as myelination must take place. These processes can occur only within a limited window of opportunity and suffer from the impact of numerous inhibitory components [4,13–15,16& ]. To improve functional recovery therapeutic approaches should, therefore, be devised by either supporting endogenous cell populations to overcome critical inhibitory impacts or by providing the inflamed or injured CNS with repair-mediating cells from outside. We here describe recent developments related to the identification of repair impediments and their biological or pharmacological neutralization. Moreover, an update is provided on recent studies on exogenous stem cell application and on how this could be translated toward more efficient myelin repair in the adult.
Fingolimod, under the trademark Gilenya, was the first oral medication approved for the treatment of RRMS [17]. This compound gained further interest as a number of preclinical studies provided evidence that apart from the effect on lymphocyte trafficking, neural cells might also benefit from sphingosine-1-phosphate receptor modulation – among them oligodendroglial survival and differentiation as well as improved remyelination [18,19]. Such findings were recently supported by the observation that also in the inflamed CNS [experimental autoimmune encephalomyelitis (EAE)], Fingolimod treatment elicited increased OPC proliferation and differentiation responses [20]. Whether this is the underlying mechanism for the observed slowing of brain atrophy in RRMS patients under Gilenya treatment (TRANSFORMS study) [21] is controversially discussed even more so as a similar reduction was not observed in PPMS patients as revealed by the INFORMS study [22& ].
A completely different mode of action is attributed to the monoclonal antibody BIIB03 as it was specifically designed to neutralize the oligodendroglial differentiation inhibitor leucine rich repeat and Immunoglobin-like domain-containing protein 1 (LINGO-1) [23]. Although blocking or downregulation of LINGO-1 was repetitively shown to boost OPC differentiation and to confer increased remyelination efficiencies in experimental models, it remains to be shown to what extent such an antibody can provide myelin repair in the deep brain parenchyma. Although in a current trial on the effect of BIIB033 in acute optic neuritis (ClinicalTrials.gov: NCT01721161) retinal nerve fiber thickness preservation (as primary endpoint) was not affected, improved nerve conduction velocity as measured by visually evoked potential recordings was found. As this secondary outcome probably mirrors functional remyelination, results of a phase 2 study in RRMS (ClinicalTrials.gov: NCT01864148) are eagerly awaited.
GNbAC1 is a humanized antibody directed against the envelope protein (ENV) of the multiple sclerosis-associated retrovirus (MSRV) also known as Human Endogenous Retrovirus type W (HERV-W) [24–27]. Although evolutionary acquired, this genetic element is thought to act as endogenous genes being mainly silenced but activated upon viral infections and/or in autoimmune conditions [28,29]. Initially discovered in leptomeningeal cells from multiple sclerosis patients [30], reactivated MSRV particles and the ENV protein were then detected in the serum and the cerebrospinal fluid of multiple sclerosis patients [31] and ENV was shown to act as a proinflammatory factor [32]. As the same viral protein was recently shown to induce oligodendroglial stress responses and to inhibit OPC differentiation [33], GNbAC1 can reverse this reaction [34& ] and raise the possibility that neutralization of HERV-W ENV might constitute yet another approach promoting remyelination. Of note, in a recent phase 2a study, this antibody was found to be well tolerated and safe [35& ,36,37], and currently a phase 2b clinical trial is initiated.
Semaphorins are a family of molecules initially described in the context of axonal growth cone repulsion and steering [38], but specific members such as Sema4D, Sema-3A and Sema-3F were found in multiple sclerosis tissue where they impact oligodendroglial cell survival, recruitment and differentiation [39–42]. Recent investigations have now shown that anti-Sema4D antibodies can attenuate EAE while preserving blood–brain barrier (BBB) integrity and axonal myelination, and to promote OPCs recruitment to lesion sites [43]. Another member of this family, Sema7A, might turn out to be a biomarker for monitoring multiple sclerosis disease progression based on descriptions on elevated titres in worsening disease courses [44&&,45]. Two further molecules previously identified in studying axonal guidance mechanisms, namely netrin-1 [46& ] and ephrinB3 [47], were detected in multiple sclerosis lesions and shown to limit OPC recruitment and their differentiation, respectively. These effects, with repeated demyelinating episodes, contribute to permanent remyelination failure.
On the basis of results of a clinical trial in which simvastatin was shown to reduce brain atrophy and disability in SPMS [48], statins might constitute further promising drugs in regard to the development of remyelination therapies. These findings may mechanistically reflect previous preclinical observations on statin-improved OPC survival, differentiation and remyelination [49,50]. However, despite the encouraging result of the clinical trial, it may appear awkward to use an inhibitor of cholesterol synthesis to reconstitute a membrane, whose major constituent is cholesterol.
Apart from these compounds, which had been studied for quite some time in the context of remyelination, additional pharmacological substances have recently been investigated. High-throughput screenings were conducted and revealed that, for example antimuscarinic compounds [51,52], the antifungal agent miconazole and the glucocorticoid clobetasol [53] or benztropine [54] act as oligodendrogliogenic agents. Moreover, the NSAID indometacin [55], histamine receptor blockers [56,57], choline metabolites [58] and the estrogen receptor b agonist indazole-Cl [59] were similarly found to exert beneficial effects to the oligodendroglial precursor cell compartment. Finally and most notably, Gli-antagonist 61 (GANT61), a specific blocker of the transcriptional regulator Gli1, was applied to mice with experimental demyelination and shown to boost adult neural stem cell-mediated remyelination [60&&]. In light of recent descriptions of a substantial contribution of such adult stem cells to myelin reconstitution [9&&], a pharmacological modulation of stem cell niche activities could open additional therapeutic avenues. A more general description of current compounds with suspected remyelination activities has been provided in a current overview article [61& ]. Moreover, innovative in-vitro, ex-vivo and in-vivo screening tools, which are described in the article by Stankoff et al. (pp. 286–292) in the same issue of Current Opinion of Neurology, have allowed identifying promising candidates with remyelinating efficacy.
EXOGENOUS CELL-BASED APPROACHES
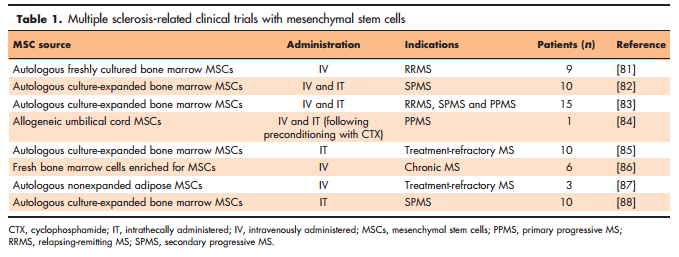
 Although various stem cell types are investigated in regard of multiple sclerosis tissue restoration [62], pharmacological modulation of neural stem cell activities, such as described above, is a new approach and probably because of increasing knowledge of stem cell inhibitory pathways [60&&,63,64]. So far, stem cells have mainly been considered in the context of transplantation and for providing either exogenous cell replacement or myelin repair via immunomodulatory or trophic activities (Fig. 1). Related to such bystander processes, the influence of mesenchymal stem cells [(MSCs); Fig. 1] on adult NSCs as well as on resident OPCs has gained much interest. Naturally, bone marrow-derived MSCs have the ability to differentiate into osteoblasts, chondrocytes and adipocytes [65] and are responsible for tissue renewal in the aged body or upon damage. Notwithstanding, MSCs can also secrete factors fostering oligodendroglial differentiation [66,67] and enhance remyelination in EAE animals [68]. Human umbilical cord-derived MSCs can also promote remyelination [69], and multiple intrathecal injections of autologous bone marrow MSCs into the EAE animals improved the disease score, increased the number of progenitors, diminished immune cell infiltration and reduced the area of demyelination [70]. Such observations are currently challenged in a phase 1 clinical trial assessing the intrathecal administration of autologous MSC-derived neural progenitors in multiple sclerosis patients (ClinicalTrials.gov: NCT01933 802). Furthermore, a phase 2a proof-of-concept study showed an improvement of visual acuity and shortening of delayed visual-evoked response latency after intravenous infusion of autologous bone marrow MSCs in SPMS patients (ClinicalTrials.gov: NCT00395200) [71]. Both clinical trials are based on preclinical data showing immunomodulatory as well as neuroprotective effects in EAE [72,73] and are particularly important for safety reasons as available data also demonstrated possible disease worsening in CD8þ T-cell-driven myelin oligodendrocyte glycoprotein-EAE (MOG-EAE) [74]. However, it cannot be ignored that MSCs mainly exert positive immunomodulatory effects such as an impairment of T-cell trafficking across the BBB [75] and induction of neuroprotective microglia phenotypes [76]. Likewise, the influence of MSCs on oligodendroglial dynamics under noninflammatory conditions has been controversially discussed. MSCs transplanted upon cuprizonemediated demyelination activated oligodendrogenesis and remyelination [77,78], whereas intravenously or intranasally applied cells did not affect the CNS [79,80]. These observations clearly emphasize the need for further investigations. An overview of multiple sclerosis-related clinical trials with MSCs is provided in Table 1 [81–88]. When considering exogenous cell replacement, alternative cell types such as aNSCs or even OPCs appear to be logical sources when to be engrafted into different CNS regions (Fig. 1). Apart from subventricular zone-derived adult NSCs, which were repetitively shown to contribute to the formation of new oligodendrocytes [8,9&&,10], targeting hippocampal NSCs and programming them into oligodendrocytes was also described [11]. Moreover, neural precursor cells are also able to modulate the immune system in EAE models when transplanted subcutaneously [89]. Not only cell differentiation must be controlled, but grafted cells need to be also successfully recruited to lesion sites. It is, therefore, of interest to note that population of inflammatory demyelinating lesions by transplanted OPCs was found to depend on cell-surface glycoprotein CD44 expression [90]. Further limitations consist of adverse astroglial differentiation of aNSCs and of the immune response directed against grafts. For instance, the modulation of chordin, microRNA- 153 and Hes6 may support transplantation efficiencies of cells as they were found to regulate astrogliogenic differentiation of NSCs [63,64,91–93].
Although various stem cell types are investigated in regard of multiple sclerosis tissue restoration [62], pharmacological modulation of neural stem cell activities, such as described above, is a new approach and probably because of increasing knowledge of stem cell inhibitory pathways [60&&,63,64]. So far, stem cells have mainly been considered in the context of transplantation and for providing either exogenous cell replacement or myelin repair via immunomodulatory or trophic activities (Fig. 1). Related to such bystander processes, the influence of mesenchymal stem cells [(MSCs); Fig. 1] on adult NSCs as well as on resident OPCs has gained much interest. Naturally, bone marrow-derived MSCs have the ability to differentiate into osteoblasts, chondrocytes and adipocytes [65] and are responsible for tissue renewal in the aged body or upon damage. Notwithstanding, MSCs can also secrete factors fostering oligodendroglial differentiation [66,67] and enhance remyelination in EAE animals [68]. Human umbilical cord-derived MSCs can also promote remyelination [69], and multiple intrathecal injections of autologous bone marrow MSCs into the EAE animals improved the disease score, increased the number of progenitors, diminished immune cell infiltration and reduced the area of demyelination [70]. Such observations are currently challenged in a phase 1 clinical trial assessing the intrathecal administration of autologous MSC-derived neural progenitors in multiple sclerosis patients (ClinicalTrials.gov: NCT01933 802). Furthermore, a phase 2a proof-of-concept study showed an improvement of visual acuity and shortening of delayed visual-evoked response latency after intravenous infusion of autologous bone marrow MSCs in SPMS patients (ClinicalTrials.gov: NCT00395200) [71]. Both clinical trials are based on preclinical data showing immunomodulatory as well as neuroprotective effects in EAE [72,73] and are particularly important for safety reasons as available data also demonstrated possible disease worsening in CD8þ T-cell-driven myelin oligodendrocyte glycoprotein-EAE (MOG-EAE) [74]. However, it cannot be ignored that MSCs mainly exert positive immunomodulatory effects such as an impairment of T-cell trafficking across the BBB [75] and induction of neuroprotective microglia phenotypes [76]. Likewise, the influence of MSCs on oligodendroglial dynamics under noninflammatory conditions has been controversially discussed. MSCs transplanted upon cuprizonemediated demyelination activated oligodendrogenesis and remyelination [77,78], whereas intravenously or intranasally applied cells did not affect the CNS [79,80]. These observations clearly emphasize the need for further investigations. An overview of multiple sclerosis-related clinical trials with MSCs is provided in Table 1 [81–88]. When considering exogenous cell replacement, alternative cell types such as aNSCs or even OPCs appear to be logical sources when to be engrafted into different CNS regions (Fig. 1). Apart from subventricular zone-derived adult NSCs, which were repetitively shown to contribute to the formation of new oligodendrocytes [8,9&&,10], targeting hippocampal NSCs and programming them into oligodendrocytes was also described [11]. Moreover, neural precursor cells are also able to modulate the immune system in EAE models when transplanted subcutaneously [89]. Not only cell differentiation must be controlled, but grafted cells need to be also successfully recruited to lesion sites. It is, therefore, of interest to note that population of inflammatory demyelinating lesions by transplanted OPCs was found to depend on cell-surface glycoprotein CD44 expression [90]. Further limitations consist of adverse astroglial differentiation of aNSCs and of the immune response directed against grafts. For instance, the modulation of chordin, microRNA- 153 and Hes6 may support transplantation efficiencies of cells as they were found to regulate astrogliogenic differentiation of NSCs [63,64,91–93].
 For a long time, pluripotent embryonic stem cells (ESCs), with their potential to differentiate into cells of all three germ layers, were thought to be ‘saviours’ among all regenerating cell types (Fig. 1). And despite the safety and ethical issues, they are still in focus when it comes to the development of new therapies for conditions with ineffective or deficient endogenous cell repair mechanisms. It is, indeed, possible to generate OPCs from human embryonic stem cells [94] and to transplant human ESC-derived OPCs into irradiated brains [95] or spinal cords [96] for functional myelin restoration. Although this requires a deep knowledge of the mechanisms involved in pluripotent stem cell differentiation, it is still unclear to what degree such processes can be controlled properly. Moreover, ethical issues remain, basically related to their origin, that is the inner cell mass of human blastocysts.
For a long time, pluripotent embryonic stem cells (ESCs), with their potential to differentiate into cells of all three germ layers, were thought to be ‘saviours’ among all regenerating cell types (Fig. 1). And despite the safety and ethical issues, they are still in focus when it comes to the development of new therapies for conditions with ineffective or deficient endogenous cell repair mechanisms. It is, indeed, possible to generate OPCs from human embryonic stem cells [94] and to transplant human ESC-derived OPCs into irradiated brains [95] or spinal cords [96] for functional myelin restoration. Although this requires a deep knowledge of the mechanisms involved in pluripotent stem cell differentiation, it is still unclear to what degree such processes can be controlled properly. Moreover, ethical issues remain, basically related to their origin, that is the inner cell mass of human blastocysts.
In this context, induced pluripotent stem cells (iPSCs) [97] could represent a valuable alternative cell model (Fig. 1). Corresponding autologous transplantation schemes come with less ethical concerns and furthermore diminish rejection reactions and the need to immunosuppress recipients. However, it remains to be shown whether the potential of iPSCs is similar to the one of ESCs and whether they could fully replace them. Furthermore, important technical issues need to be solved, among them the establishment of efficient protocols for the generation of oligodendroglial cells. iPSC-dependent oligodendrogenesis was shown to include a radialglia intermediate step as revealed by the expression of paired box protein 6 followed by a transition into OLIG2-positive and NKX2.2-positive cells, massive cell proliferation and subsequent expression of myelin proteins [98]. However, the generation of human iPSC-derived oligodendroglia is timeconsuming and first protocols employed 200 days of culturing before myelin markers were expressed [99]. Despite this technical drawback, such cells were nevertheless shown to generate myelin-forming oligodendrocytes upon transplantation into demyelinated lesions [100,101]. In practice, though, even a direct conversion of fibroblasts into OPCs was sufficient to allow myelin generation after engraftment into shiverer mutant mice [102]. Moreover, human iPSC-derived OPCs are also able to myelinate axons within 24 h after transplantation into injured spinal cords [103]. When comparing reprogrammed mouse skin-derived fibroblasts to mouse CNS-derived neural precursors, both cell types revealed similar differentiation capacities, tissue integration and myelin formation properties [104] highlighting the great potential of reprogrammed cells. Importantly, a substantial step toward multiple sclerosis repair was made by a proof-of-concept study demonstrating that autologous transplantation of iPSC-derived OPCs from PPMS patients might be practicable as such cells myelinated axons of shiverer mice [105]. Nonetheless, improved protocols need to be developed that assure a shorter time course. A recently published procedure indeed described oligodendroglial differentiation within 75 days [106]. Another important question to be solved is to what degree such cells retain an increased tumorigenic potential based on their modulation via oncogenic factors. Although Wang et al. [100] did not detect any tumors within a 9-month period post grafting of human iPSC-derived OPCs into myelin-deficient shiverer mice, further studies explored the underlying mechanisms of carcinoma formation [107–110]. In a similar vein, migration of transplanted cells appears to be limited but was found to be stimulated following overexpression of the polysialylated neural cell adhesion molecule concomitantly with the generation of myelin in demyelinated corpus callosum [111]. Finally, yet another advantage of engrafting reprogrammed cells has surfaced through the observation that they can participate in remyelination by promoting survival, differentiation and remyelination of endogenous oligodendroglial cells [112]. However, intracerebral transplantation raises the question of the production of a sufficient large number of cells with good manufacturing practice quality, and the neurosurgical risk.
CONCLUSION
It has been recognized that while ever more effective immunomodulatory treatments of patients with RRMS provide significant benefit, long-term improvement will depend on the generation of neuroprotective and repair therapies. A number of novel aspects related to endogenous as well as exogenous regeneration mechanisms are currently explored in detail that hold promise in reversing disability and improving patient’s quality of life.
However, it remains to be shown to what degree the modulation of intrinsic mechanisms can be successfully applied, whether all multiple sclerosis lesions retain responsiveness to repair attempts and whether the application of exogenous repairmediating cells turns out to be superior and how they can efficiently be delivered to demyelinated areas. Moreover, in times of first clinical studies, assessing repair activities efforts must be undertaken to improve and refine imaging techniques and search for suitable biomarkers in order not to miss out on glioprotective and regenerative effects.
Financial support and sponsorship
Research on the subject of myelin repair in the German laboratory is supported by the German Research Council (DFG: SPP1757_KU1934/2–1, KU1934/5–1), the Christiane and Claudia Hempel Foundation for clinical stem cell research and the French research foundations ARSEP and AFM. The MS Center at the Department of Neurology is supported in part by the Walter and Ilse Rose Foundation and the James and Elisabeth Cloppenburg, Peek and Cloppenburg Du¨sseldorf Foundation. Research on the subject of myelin repair in the French laboratory work is supported by INSERM, the French MS research foundation ARSEP, the program ‘Investissements d’avenir’ ANR-10- IAIHU-06, a grant from ‘Investissement d’Avenir – ANR- 11-INBS-0011’ – NeurATRIS’ and ANR grants STEMIMUS to CL and OLGA to BZ.
Conflicts of interest
J.J.J. reports no conflicts of interest. H.P.H. received compensation for consultancy and speaking from Biogen, GeNeuro, Genzyme, MerckSerono, Novartis, Octapharma, Opexa, Receptos, Roche, Teva and Sanofi. P.K. received compensation for consultancy and/or speaking from Novartis, Baxter and GeNeuro, and research support from Genzyme and Baxter. C.L. has received honoraria from Roche, Biogen, Genzyme and Novartis, and participated to Vertex advisory board. B.Z. has received research grants from Novartis and EMD Serono. B.S. has received honoraria from Biogen, Teva, Novartis and Genzyme, and research support from Genzyme and Merck-Serono.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
& of special interest
&& of outstanding interest
1. Morrison BM, Lee Y, Rothstein JD. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol 2013; 23:644–651.
2. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015;
14:183–193. 3. Aktas O, Kieseier B, Hartung HP. Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci 2010; 33:140–152.
4. Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 2002; 346:165–173.
5. Baker D, Amor S. Mouse models of multiple sclerosis: lost in translation? Curr Pharm Des 2015; 21:2440–2452.
6. & Crawford AH, Tripathi RB, Foerster S, et al. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am J Pathol 2016; 186:511–516. Strong experimental evidence on the previous assumption that fully matured oligodendrocytes do not participate in myelin repair activities.
7. Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 2000; 20:6404–6412.
8. Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A 2007; 104:4694–4699.
9. && Xing YL, Roth PT, Stratton JA, et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci 2014; 34:14128–14146. In this recent article, a significant contribution of adult NSCs from the subventricular zone to remyelination was demonstrated.
10. Picard-Riera N, Decker L, Delarasse C, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 2002; 99:13211–13216.
11. Jessberger S, Toni N, Clemenson GD Jr, et al. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 2008; 11:888–893.
12. van Niekerk EA, Tuszynski MH, Lu P, Dulin JN. Molecular and cellular mechanisms of axonal regeneration after spinal cord injury. Mol Cell Proteomics 2016; 15:394–408.
13. Kuhlmann T, Miron V, Cuo Q, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008; 131 (Pt 7):1749–1758. 14. Kremer D, Aktas O, Hartung HP, Ku¨ry P. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol 2011; 69:602–618.
15. Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease: can we wrap it up? Brain 2011; 134 (Pt 7):1882–1900.
16. & Go¨ttle P, Sabo JK, Heinen A, et al. Oligodendroglial maturation is dependent on intracellular protein shuttling. J Neurosci 2015; 35:906–919. A study highlighting the importance of intracellular protein shuttling and distribution in regulating oligodendroglial differentiation.
17. Ingwersen J, Aktas O, Ku¨ry P, et al. Fingolimod in multiple sclerosis: mechanisms of action and clinical efficacy. Clin Immunol 2012; 142:15–24.
18. Miron VE, Jung CG, Kim HJ, et al. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol 2008; 63:61–71.
19. Miron VE, Ludwin SK, Darlington PJ, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 2010; 176:2682–2694.
20. Zhang J, Zhang ZG, Li Y, et al. Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiol Dis 2015; 76:57–66.
21. Barkhof F, de Jong R, Sfikas N, et al. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler 2014; 20:1704–1713.
22. & Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, doubleblind, placebo-controlled trial. Lancet 2016; doi: 10.1016/S0140- 6736(15)01314-8. [Epub ahead of print] In patients with primary progressive multiple sclerosis Fingolimod/Gilenya, treatment did not slow brain atrophy.
23. Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs 2013; 27:493–503.
24. Curtin F, Perron H, Kromminga A, et al. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs 2015; 7:265–275.
25. Perron H, Bernard C, Bertrand JB, et al. Endogenous retroviral genes, herpes viruses and gender in multiple sclerosis. J Neurol Sci 2009; 286:65–72.
26. Dolei A, Uleri E, Ibba G, et al. The aliens inside human DNA: HERV-W/MSRV/ syncytin-1 endogenous retroviruses and neurodegeneration. J Infect Dev Ctries 2015; 9:577–587.
27. Dolei A, Perron H. The multiple sclerosis-associated retrovirus and its HERVW endogenous family: a biological interface between virology, genetics, and immunology in human physiology and disease. J Neurovirol 2009; 15:4–13.
28. Mameli G, Madeddu G, Mei A, et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: the missing link with multiple sclerosis? PLoS One 2013; 8:e78474.
29. Uleri E, Mei A, Mameli G, et al. HIV Tat acts on endogenous retroviruses of the W family and this occurs via Toll-like receptor 4: inference for neuroAIDS. AIDS 2014; 28:2659–2670.
30. Perron H, Lalande B, Gratacap B, et al. Isolation of retrovirus from patients with multiple sclerosis. Lancet 1991; 337:862–863.
31. Perron H, Germi R, Bernard C, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler 2012; 18:1721–1736.
32. Rolland A, Jouvin-Marche E, Viret C, et al. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/ TLR4 and promotes Th1-like responses. J Immunol 2006; 176:7636–7644.
33. Kremer D, Schichel T, Fo¨rster M, et al. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol 2013; 74:721–732.
34. & Kremer D, Fo¨rster M, Schichel T, et al. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation blockade. Mult Scler 2015; 21:1200–1203. First experimental evidence that neutralization of HERV-W/MSRV envelope proteins not only affects inflammation but could also rescues myelin repair processes.
35. & Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult Scler 2015; 21:885–893. Report on the outcome of a clinical trial for the HERV-W ENV protein neutralizing antibody GNbAC1.
36. Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients: a twelve month follow-up. J Neuroimmunol 2015; 285:68–70. 37. Zimmermann M, Sanderson NS, Rasenack M, et al. Immunologic monitoring during a phase 2a trial of the GNbAC1 antibody in patients with MS. Neurol Neuroimmunol Neuroinflamm 2015; 2:e144. 38. TrepsL, Le Guelte A, Gavard J. EmergingrolesofSemaphorins in theregulation of epithelial and endothelial junctions. Tissue Barriers 2013; 1:e23272. 39. Williams A, Piaton G, Aigrot MS, et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 2007; 130 (Pt 10):2554–2565.
40. Giraudon P, Vincent P, Vuaillat C, et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol 2004; 172:1246–1255.
41. Piaton G, Aigrot MS, Williams A, et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 2011; 134 (Pt 4):1156–1167.
42. Syed YA, Hand E, Mobius W, et al. Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci 2011; 31:3719–3728. 43. Smith ES, Jonason A, Reilly C, et al. SEMA4D compromises blood-brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol Dis 2015; 73:254–268.
44. && Costa C, Martinez-Saez E, Gutierrez-Franco A, et al. Expression of semaphorin 3A, semaphorin 7A and their receptors in multiple sclerosis lesions. Mult Scler 2015; 21:1632–1643. Description of Sema7A as a potential biomarker for multiple sclerosis progression.
45. Kremer D, Hartung HP, Ku¨ry P. Targeting semaphorins in MS as a treatment strategy to promote remyelination: a tale of mice, rats and men. Mult Scler 2015; 21:1616–1617.
46. & Tepavcevic V, Kerninon C, Aigrot MS, et al. Early netrin-1 expression impairs central nervous system remyelination. Ann Neurol 2014; 76:252–268. A strong experimental example on how molecules previously identified in the context of axonal growth and regeneration can disturb remyelination.
47. Syed YA, Zhao C, Mahad D, et al. Antibody-mediated neutralization of myelinassociated EphrinB3 accelerates CNS remyelination. Acta Neuropathol 2016; 131:281–298.
48. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MSSTAT): a randomised, placebo-controlled, phase 2 trial. Lancet 2014; 383:2213–2221.
49. Miron VE, Rajasekharan S, Jarjour AA, et al. Simvastatin regulates oligodendroglial process dynamics and survival. Glia 2007; 55:130–143.
50. Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol 2009; 174:1880–1890.
51. Mei F, Fancy SP, Shen YA, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 2014; 20:954–960.
52. Abiraman K, Pol SU, O’Bara MA, et al. Antimuscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J Neurosci 2015; 35:3676–3688. 53. Najm FJ, Madhavan M, Zaremba A, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015; 522:216–220. 54. Deshmukh VA, Tardif V, Lyssiotis CA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature 2013; 502:327–332.
55. Preisner A, Albrecht S, Cui QL, et al. Nonsteroidal anti-inflammatory drug indometacin enhances endogenous remyelination. Acta Neuropathol 2015; 130:247–261.
56. Saligrama N, Case LK, del Rio R, et al. Systemic lack of canonical histamine receptor signaling results in increased resistance to autoimmune encephalomyelitis. J Immunol 2013; 191:614–622.
57. Liu ZL, Wu X, Luo YJ, et al. Signaling mechanism underlying the histaminemodulated action of hypoglossal motoneurons. J Neurochem 2016. (in press). doi: 10.1111/jnc.13548.
58. Skripuletz T, Manzel A, Gropengiesser K, et al. Pivotal role of choline metabolites in remyelination. Brain 2015; 138 (Pt 2):398–413.
59. Moore SM, Khalaj AJ, Kumar S, et al. Multiple functional therapeutic effects of the estrogen receptor beta agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A 2014; 111:18061–18066.
60. && Samanta J, Grund EM, Silva HM, et al. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015; 526:448–452. Experimental description on the role of the transcription factor Gli1 in blocking neural stem cell contribution to myelin repair.
61. & Kremer D, Go¨ttle P, Hartung H-P, Ku¨ry P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci 2016. (in press). Most recent update on all current approaches investigated clinically and preclinically in the context of myelin repair. Moreover, a discussion of further diseases with white matter impact is provided.
62. Jadasz JJ, Aigner L, Rivera FJ, Ku¨ry P. The remyelination Philosopher’s Stone: stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res 2012; 349:331–347. 63. Jadasz JJ, Rivera FJ, Taubert A, et al. p57kip2 regulates glial fate decision in adult neural stem cells. Development 2012; 139:3306–3315.
64. Schmidt-Edelkraut U, Hoffmann A, Daniel G, Spengler D. Zac1 regulates astroglial differentiation of neural stem cells through Socs3. Stem Cells 2013; 31:1621–1632.
65. Cordeiro-Spinetti E, de Mello W, Trindade LS, et al. Human bone marrow mesenchymal progenitors: perspectives on an optimized in vitro manipulation. Front Cell Dev Biol 2014; 2:7.
66. Jadasz JJ, Kremer D, Go¨ttle P, et al. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One 2013; 8:e71814.
67. Lindsay SL, Johnstone SA, Mountford JC, et al. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia 2013; 61:368–382.
68. Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci 2012; 15:862–870.
69. Liu R, Zhang Z, Lu Z, et al. Human umbilical cord stem cells ameliorate experimental autoimmune encephalomyelitis by regulating immunoinflammation and remyelination. Stem Cells Dev 2013; 22:1053–1062.
70. Harris VK, Yan QJ, Vyshkina T, et al. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci 2012; 313:167– 177.
71. Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an openlabel phase 2a proof-of-concept study. Lancet Neurol 2012; 11:150–156.
72. Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells as treatment for MS: progress to date. Mult Scler 2013; 19:515–519.
73. Laroni A, Rosbo NK, Uccelli A. Mesenchymal stem cells for the treatment of neurological diseases: immunoregulation beyond neuroprotection. Immunol Lett 2015; 168:183–190. 74. Glenn JD, Smith MD, Calabresi PA, Whartenby KA. Mesenchymal stem cells differentially modulate effector CD8þ T cell subsets and exacerbate experimental autoimmune encephalomyelitis. Stem Cells 2014; 32:2744– 2755.
75. Benvenuto F, Voci A, Carminati E, et al. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther 2015; 6:245.
76. Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells 2012; 30:2044–2053.
77. Jaramillo-Merchan J, Jones J, Ivorra JL, et al. Mesenchymal stromal-cell transplants induce oligodendrocyte progenitor migration and remyelination in a chronic demyelination model. Cell Death Dis 2013; 4:e779.
78. El-Akabawy G, Rashed LA. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a nonimmune model of demyelination. Ann Anat 2015; 198:11–20.
79. Salinas Tejedor L, Berner G, Jacobsen K, et al. Mesenchymal stem cells do not exert direct beneficial effects on CNS remyelination in the absence of the peripheral immune system. Brain Behav Immun 2015; 50:155– 165.
80. Nessler J, Benardais K, Gudi V, et al. Effects of murine and human bone marrow-derived mesenchymal stem cells on cuprizone induced demyelination. PLoS One 2013; 8:e69795.
81. Llufriu S, Sepulveda M, Blanco Y, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One 2014; 9:e113936.
82. Connick P, Kolappan M, Patani R, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pretest: posttest study with blinded outcome assessments. Trials 2011; 12:62.
83. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010; 67:1187–1194.
84. Liang J, Zhang H, Hua B, et al. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler 2009; 15:644–646.
85. Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iranian J Immunol 2007; 4:50–57.
86. Rice CM, Mallam EA, Whone AL, et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther 2010; 87:679–685.
87. Riordan NH, Ichim TE, Min WP, et al. Nonexpanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 2009; 7:29. 88. Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 2010; 227:185–189.
89. Ravanidis S, Poulatsidou KN, Lagoudaki R, et al. Subcutaneous transplantation of neural precursor cells in experimental autoimmune encephalomyelitis reduces chemotactic signals in the central nervous system. Stem Cells Transl Med 2015; 4:1450–1462.
90. Piao JH, Wang Y, Duncan ID. CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia 2013; 61:361–367.
91. Jhas S, Ciura S, Belanger-Jasmin S, et al. Hes6 inhibits astrocyte differentiation and promotes neurogenesis through different mechanisms. J Neurosci 2006; 26:11061–11071.
92. Tsuyama J, Bunt J, Richards LJ, et al. MicroRNA-153 regulates the acquisition of gliogenic competence by neural stem cells. Stem Cell Rep 2015; 5:365–377.
93. Jablonska B, Aguirre A, Raymond M, et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci 2010; 13:541–550.
94. Stacpoole SR, Spitzer S, Bilican B, et al. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Rep 2013; 1:437–450.
95. Piao J, Major T, Auyeung G, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 2015; 16:198–210.
96. Nistor GI, Totoiu MO, Haque N, et al. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 2005; 49:385–396.
97. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–676.
98. Gorris R, Fischer J, Erwes KL, et al. Pluripotent stem cell-derived radial glialike cells as stable intermediate for efficient generation of human oligodendrocytes. Glia 2015; 63:2152–2167.
99. Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development 2015; 142:3983–3995.
100. Wang S, Bates J, Li X, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 2013; 12:252–264.
101. Kawabata S, Takano M, Numasawa-Kuroiwa Y, et al. Grafted human iPS cellderived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep 2016; 6:1–8.
102. Yang N, Zuchero JB, Ahlenius H, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol 2013; 31:434–439.
103. All AH, Gharibani P, Gupta S, et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One 2015; 10:e0116933.
104. Mozafari S, Laterza C, Roussel D, et al. Skin-derived neural precursors competitively generate functional myelin in adult demyelinated mice. J Clin Invest 2015; 125:3642–3656.
105. Douvaras P, Wang J, Zimmer M, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep 2014; 3:250–259.
106. Douvaras P, Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat Protoc 2015; 10:1143– 1154.
107. Lin SL, Ying SY. Mechanism and method for generating tumor-free iPS cells using intronic microRNA miR-302 induction. Methods Mol Biol 2013; 936:295–312.
108. Itakura G, Kobayashi Y, Nishimura S, et al. Controlling immune rejection is a fail-safe system against potential tumorigenicity after human iPSC-derived neural stem cell transplantation. PLoS One 2015; 10:e0116413.
109. Nori S, Okada Y, Nishimura S, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep 2015; 4:360–373.
110. Hemmer K, Zhang M, van Wullen T, et al. Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Rep 2014; 3:423–431.
111. Czepiel M, Leicher L, Becker K, et al. Overexpression of polysialylated neural cell adhesion molecule improves the migration capacity of induced pluripotent stem cell-derived oligodendrocyte precursors. Stem Cells Transl Med 2014; 3:1100–1109.
112. Laterza C, Merlini A, De Feo D, et al. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun 2013; 4:2597.

